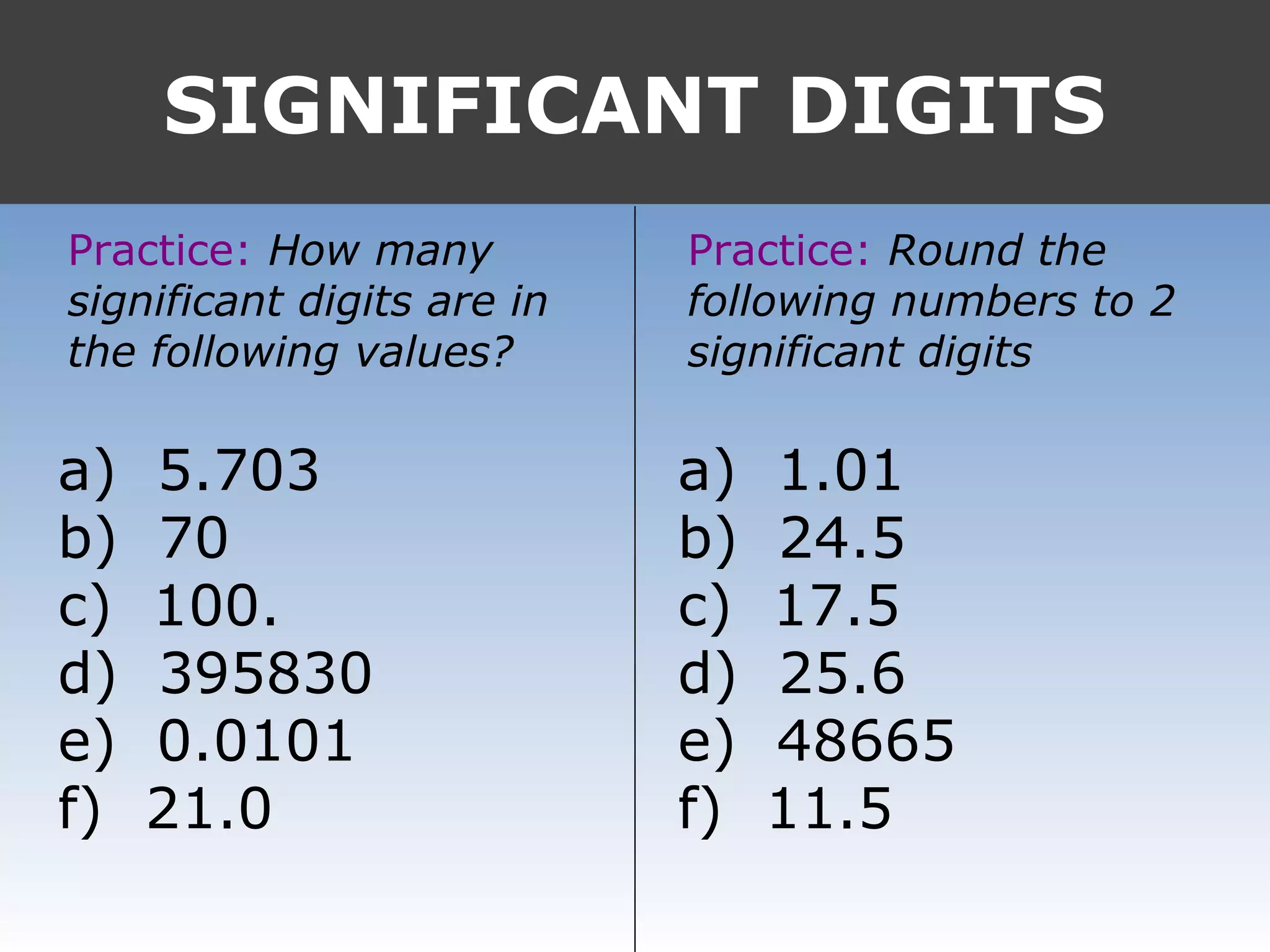
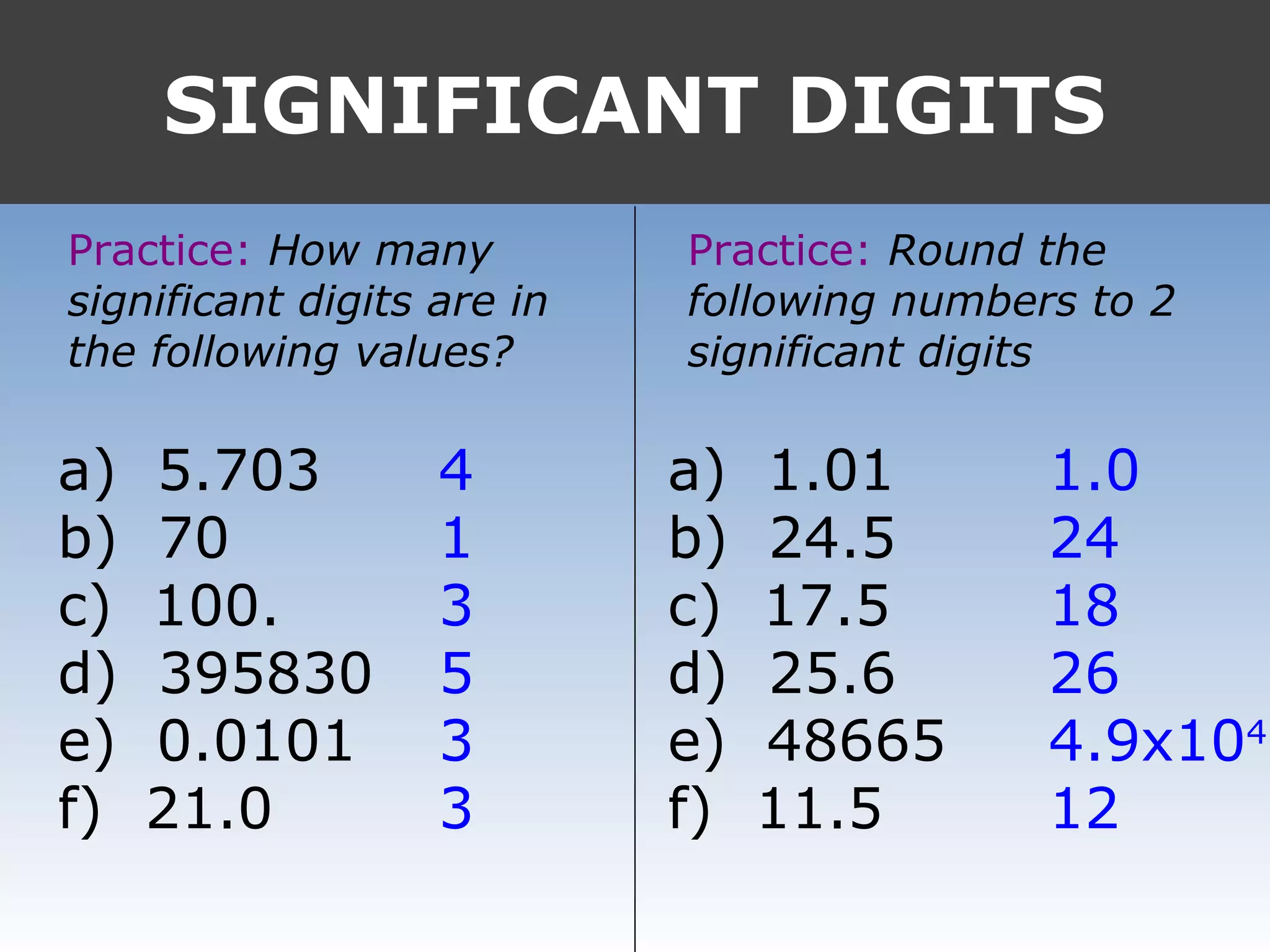
This document discusses the rules for determining significant digits in measurements and calculations. It defines four main rules:
1. All non-zero digits are significant, as are zeros between non-zero digits.
2. Leading zeros are not significant.
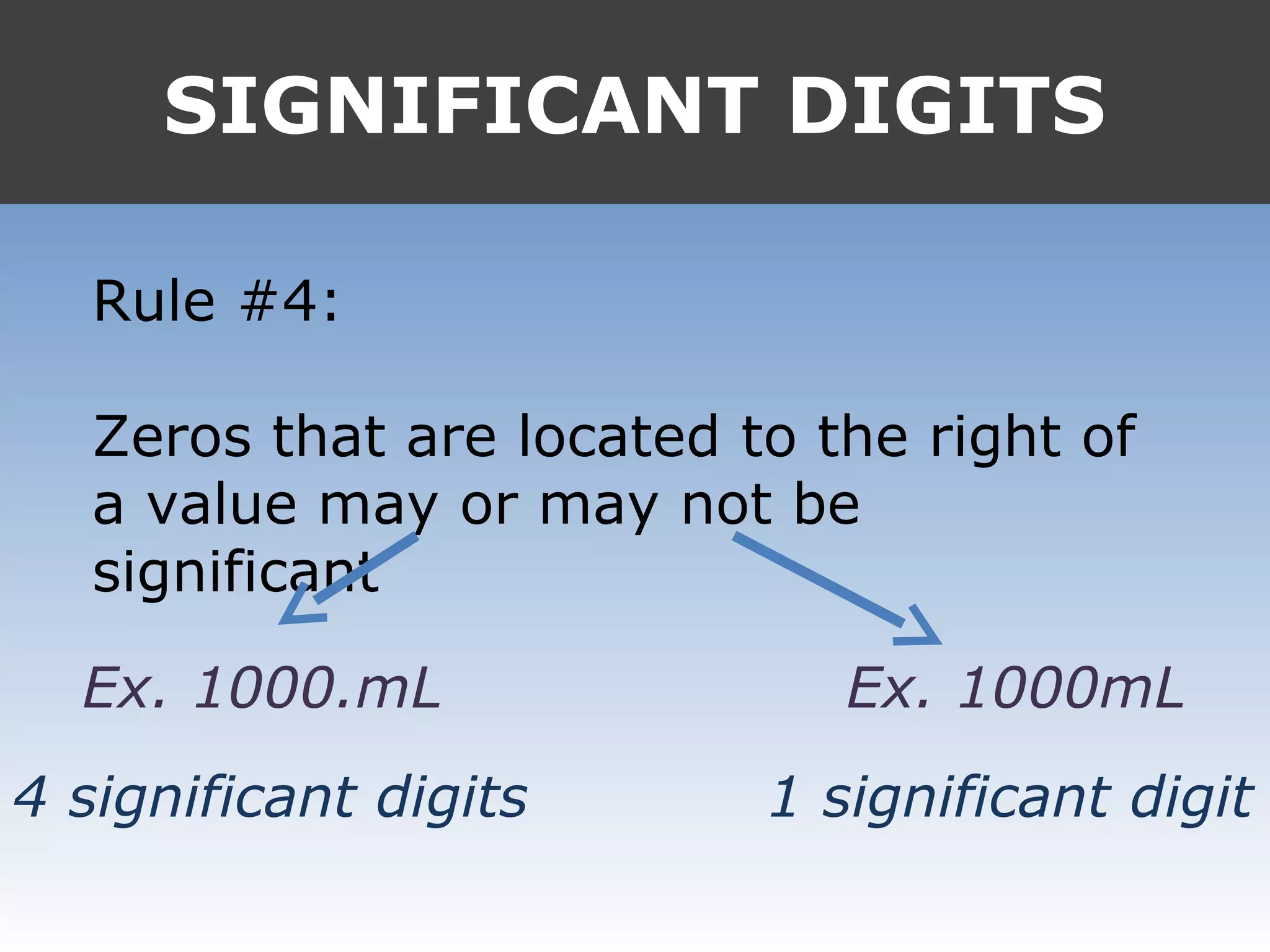
3. Trailing zeros may or may not be significant, depending on whether a decimal is present.
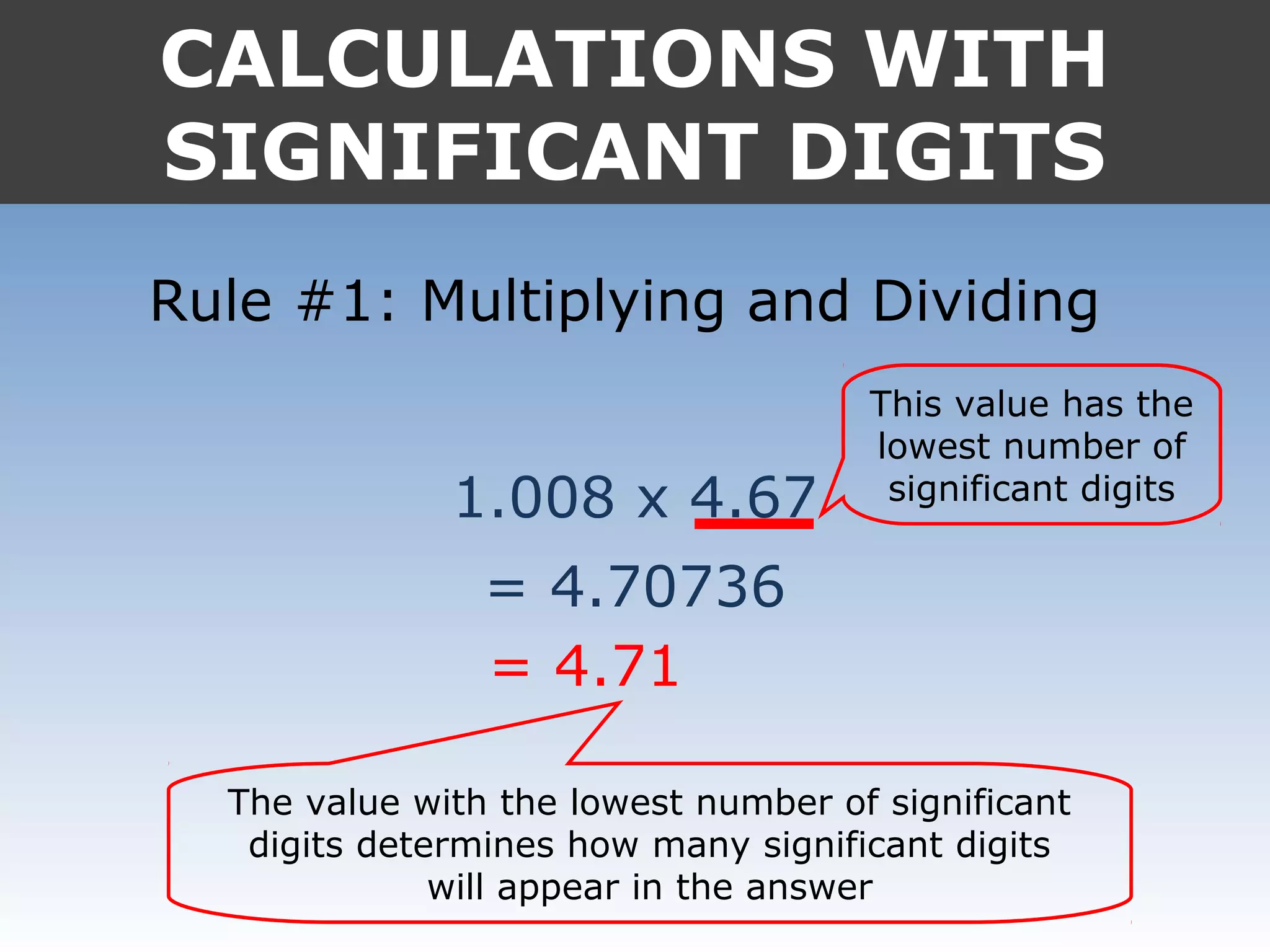
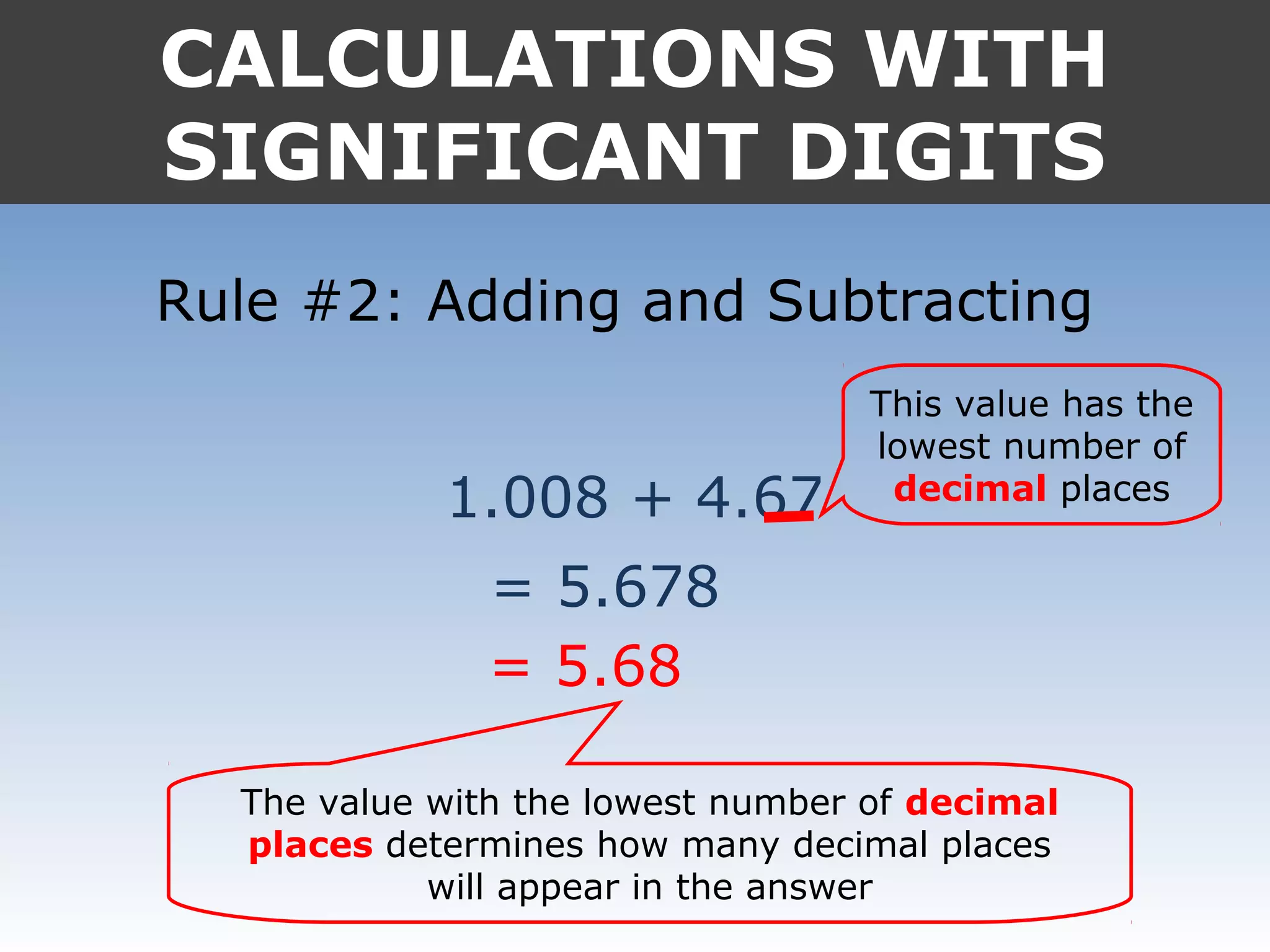
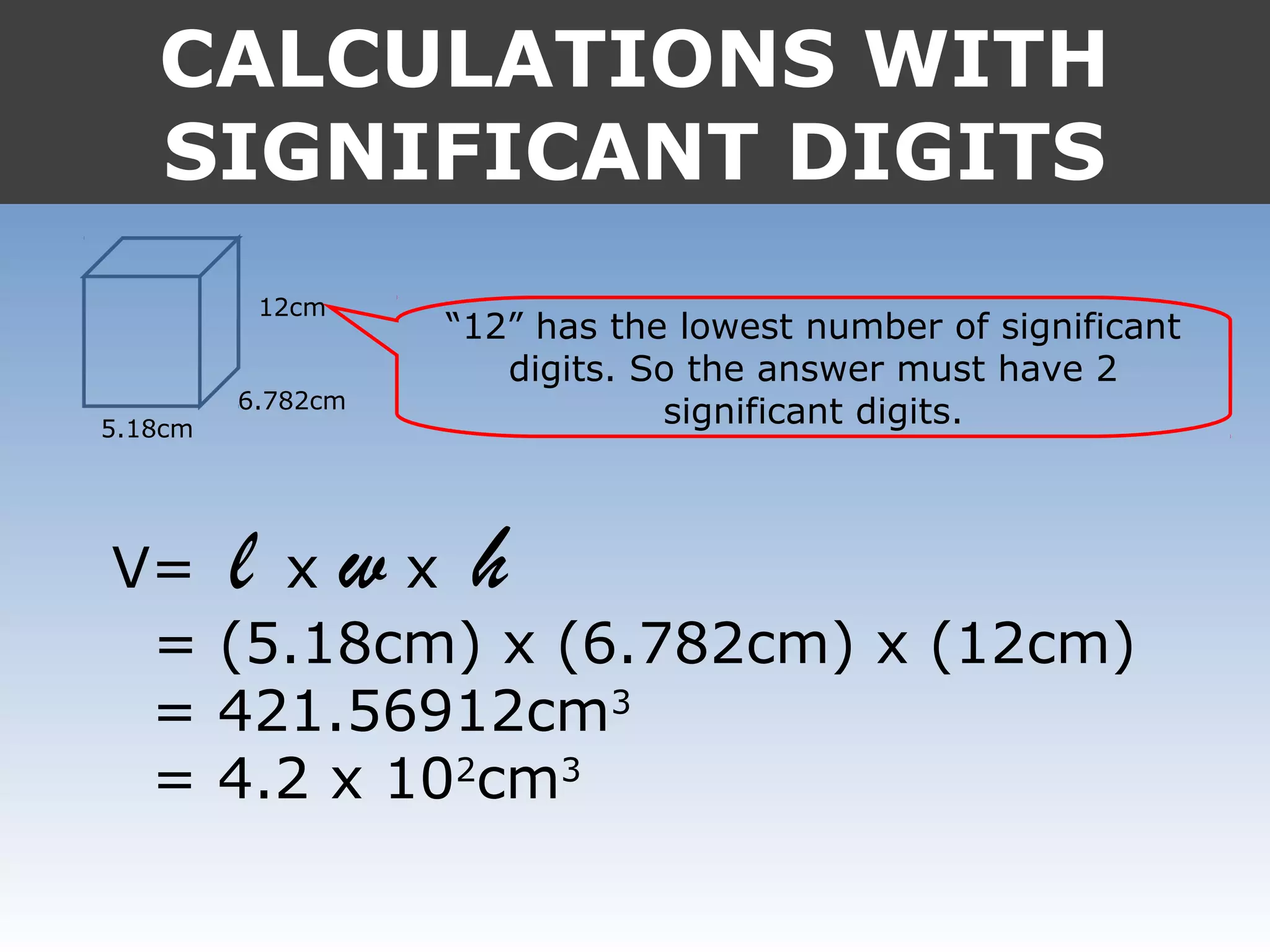
4. In calculations, the answer should match the least number of significant digits in the input values.
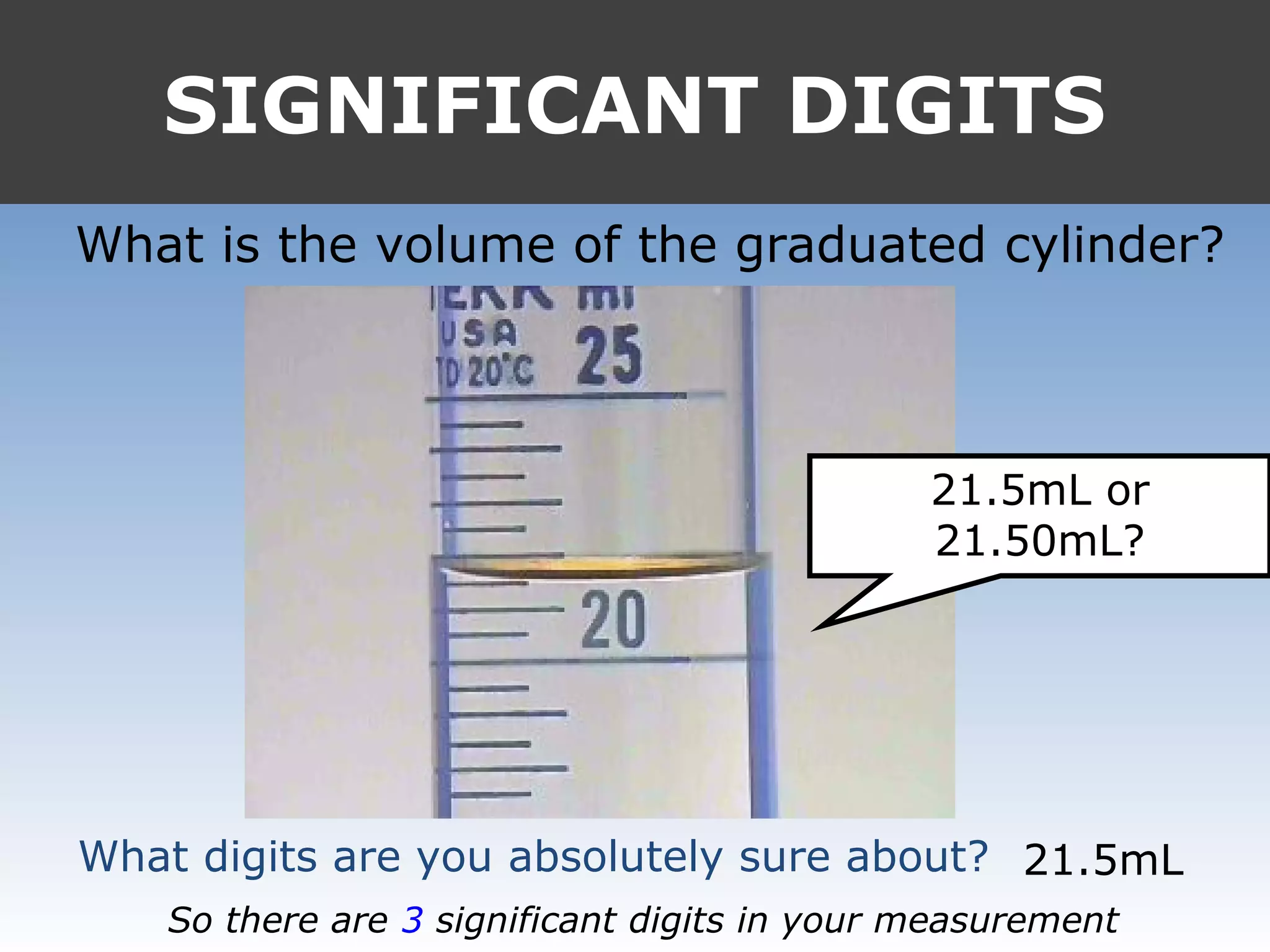
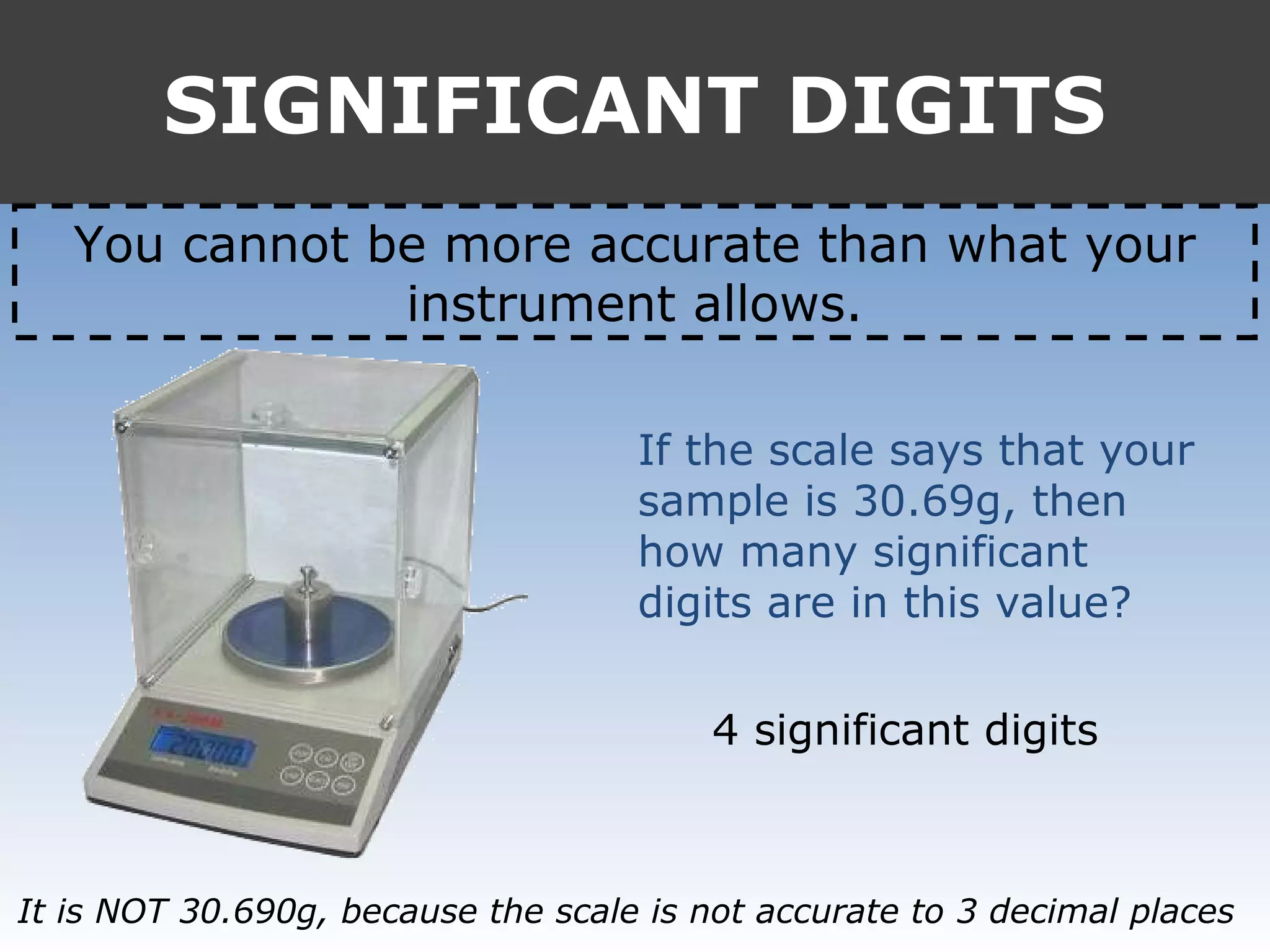
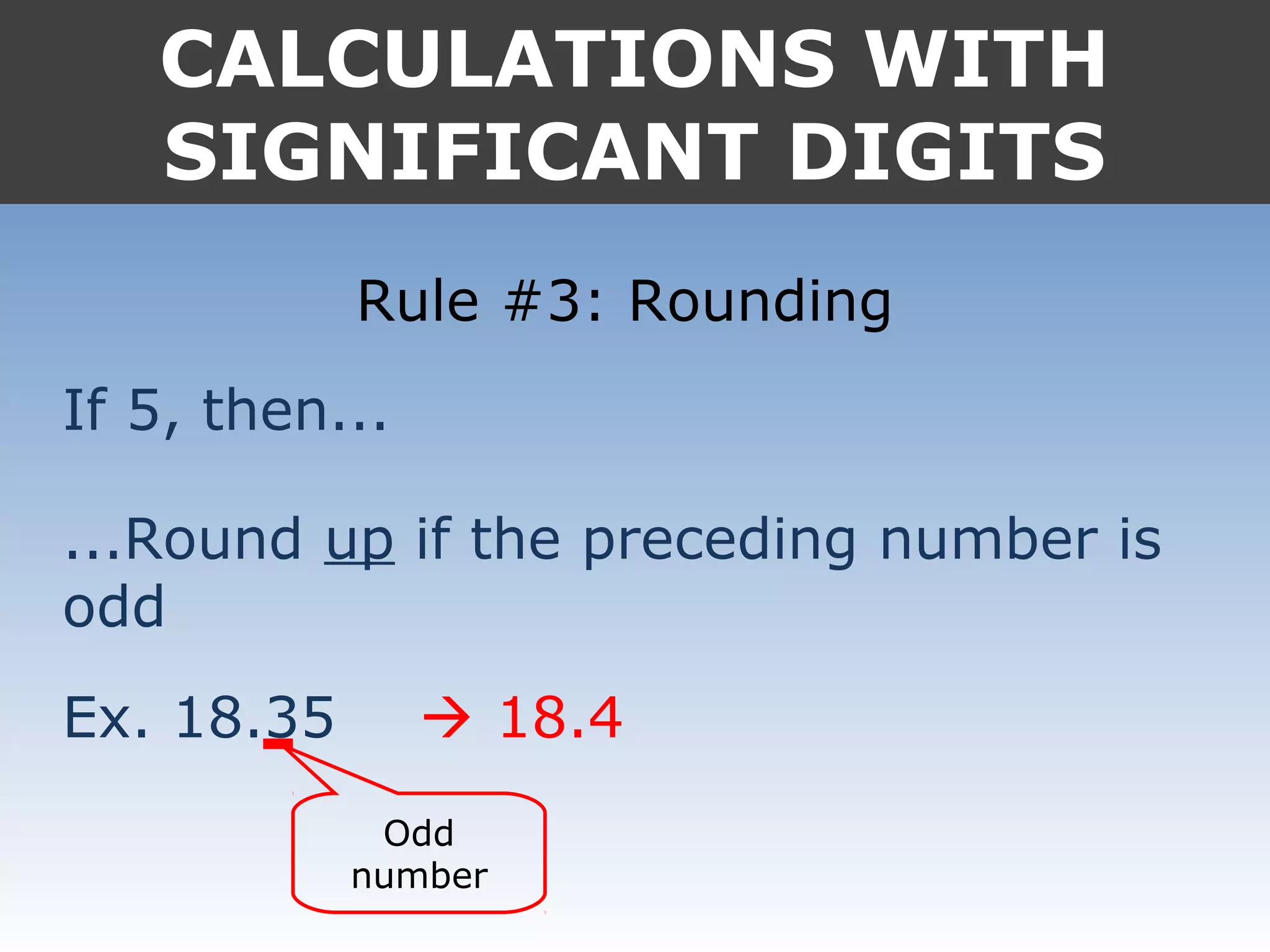
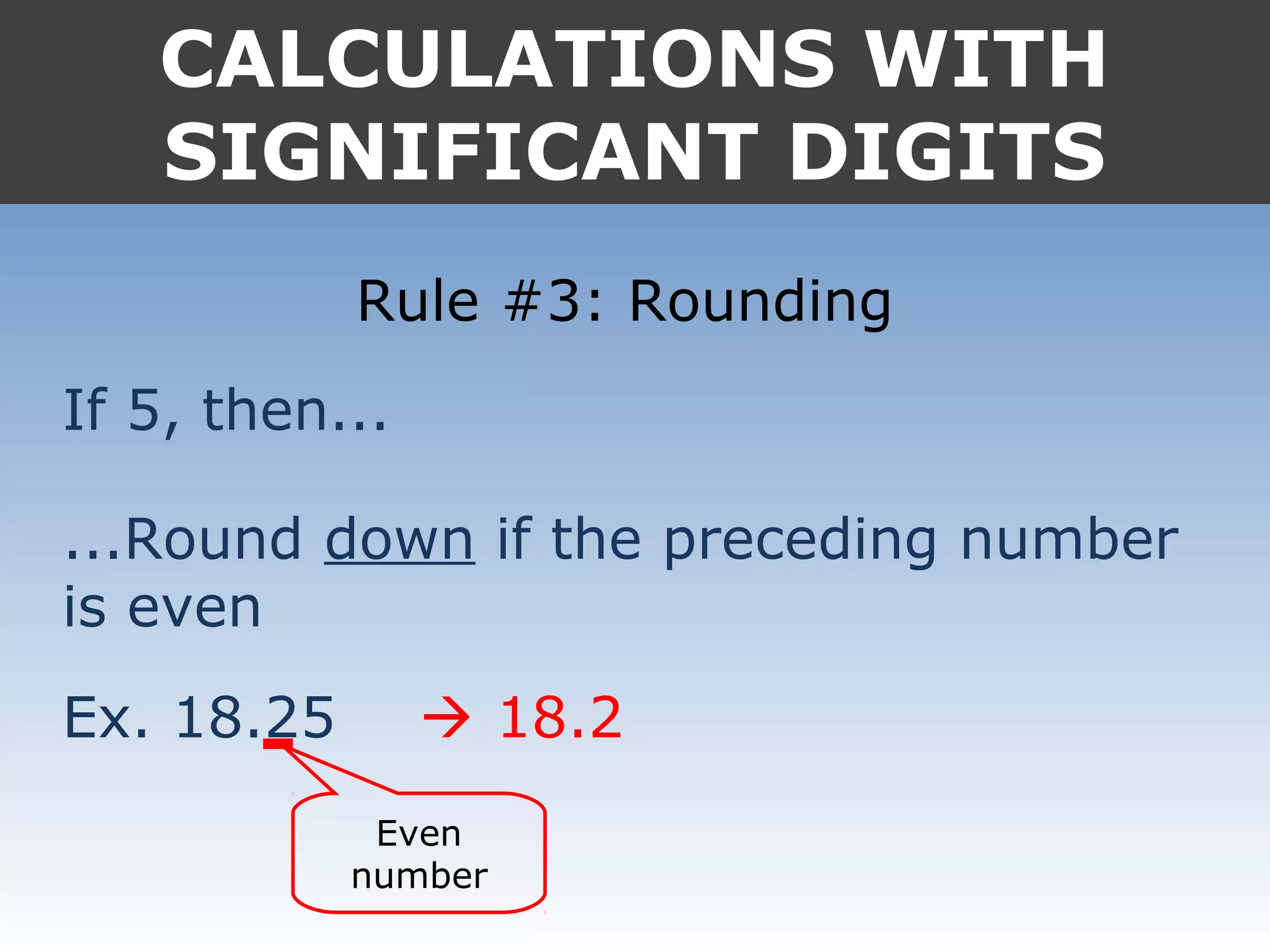
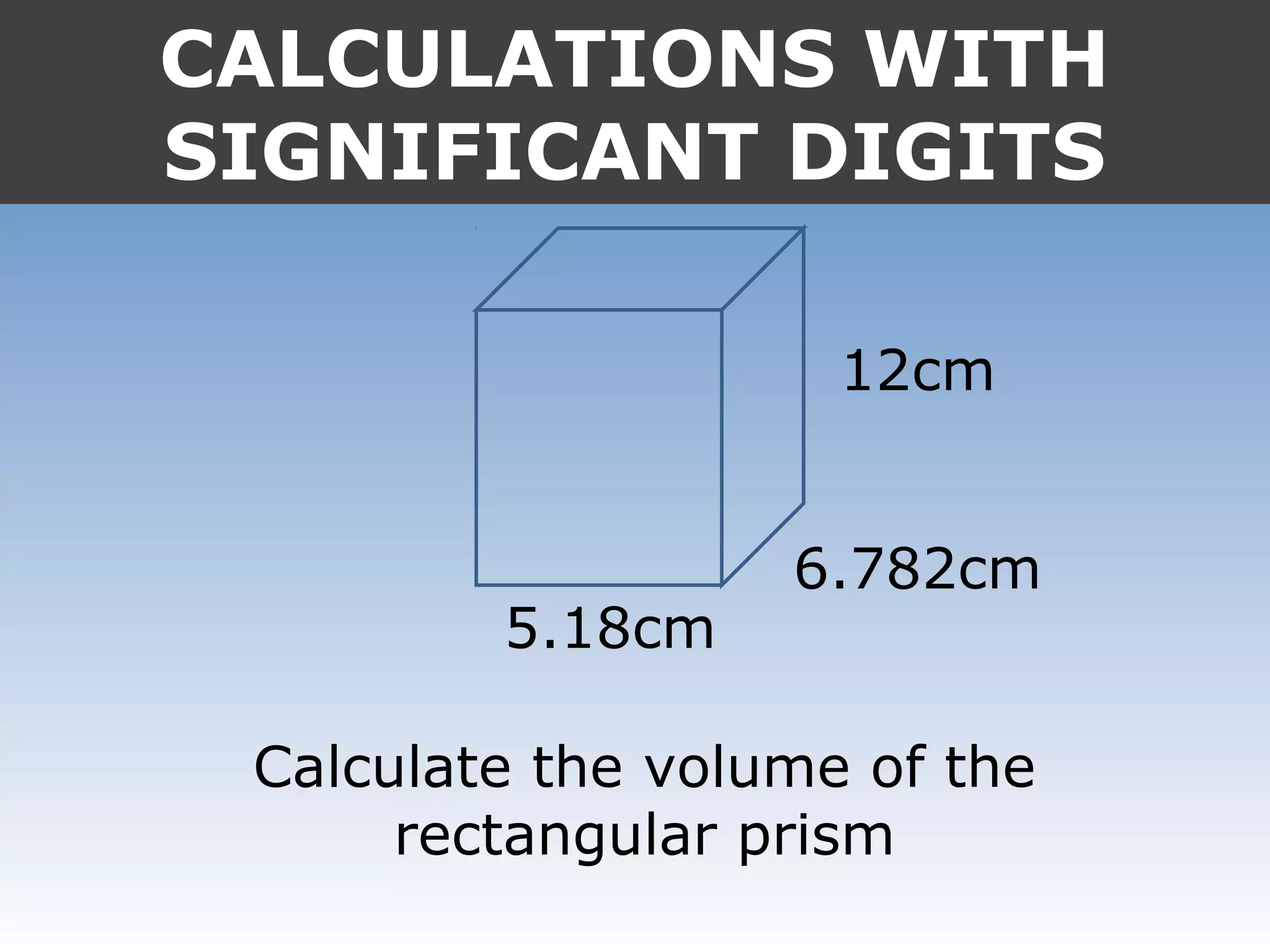
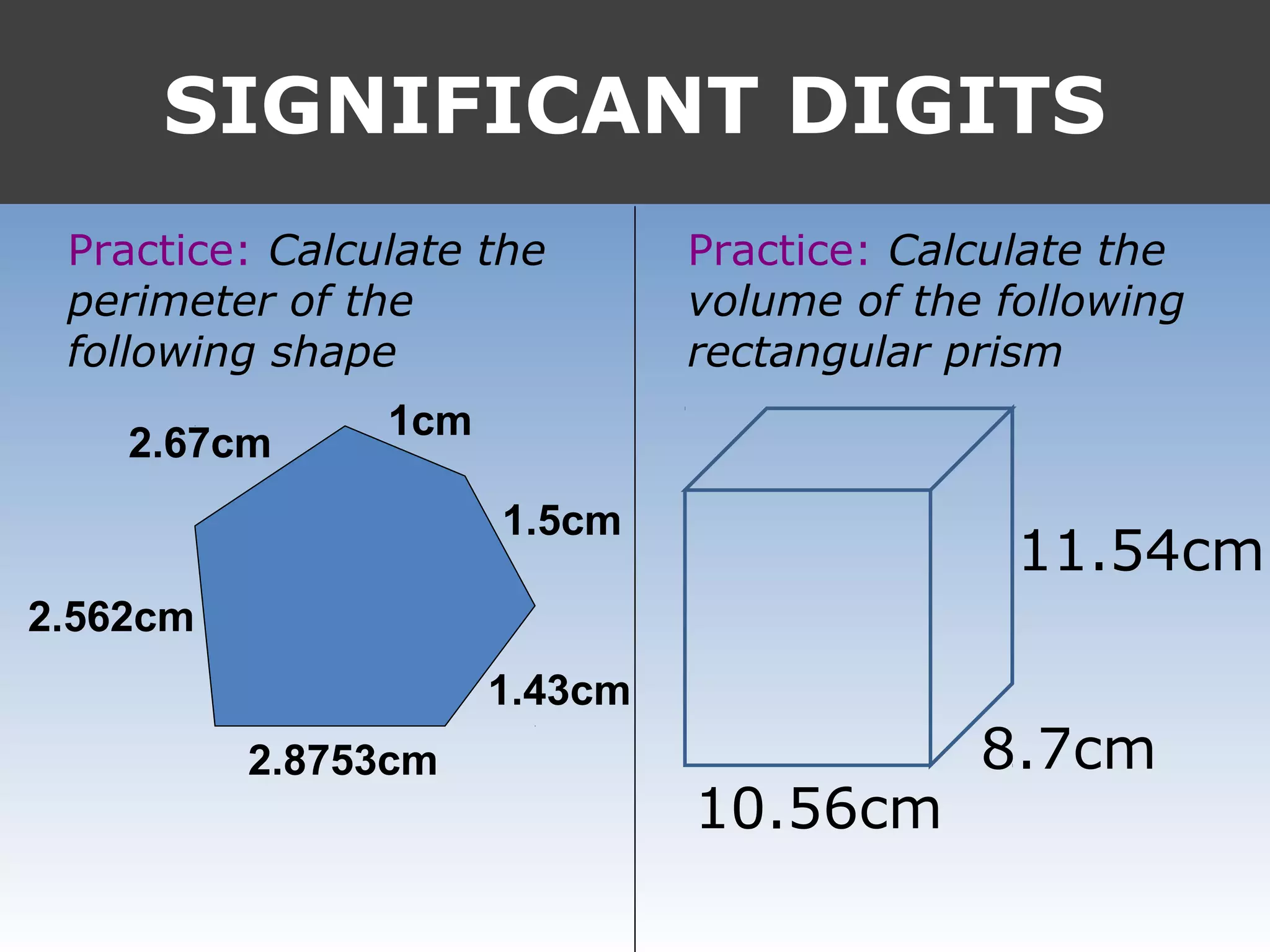
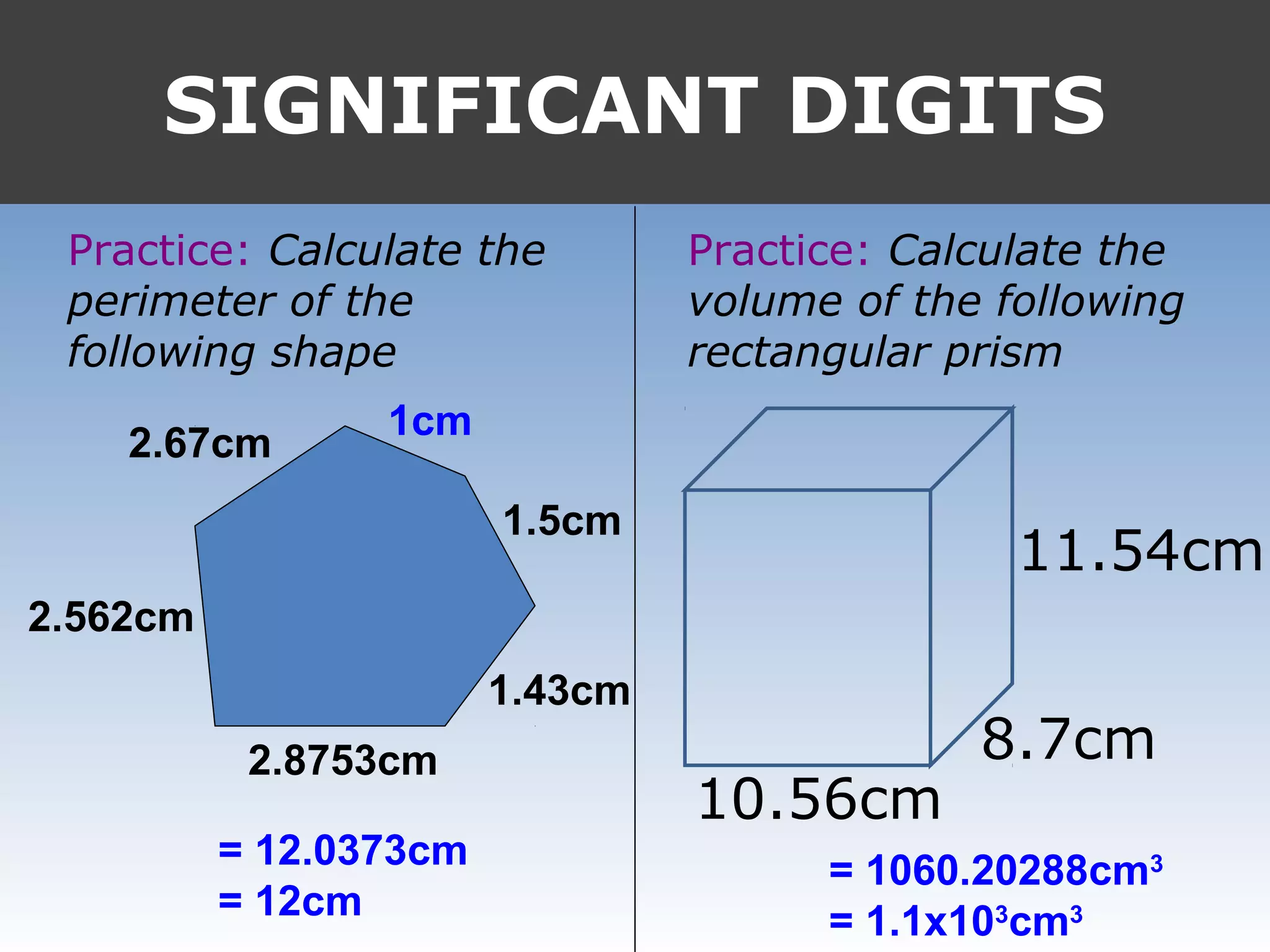
It provides examples like 0.08006 having 4 significant digits, and 1000mL having 1 significant digit. The document also covers rounding rules and calculating volumes and perimeters based on measurements to the correct number of significant digits.







![SIGNIFICANT DIGITS
Significant digits for logarithmic values:
If a solution had an H+
concentration of 2.5x10-4
mol/L,
then what is the pH to the correct number of significant
digits?
i.e. pH = - log [2.5x10-4
mol/L]
pH = 3.602059
These two digits are significant
This digit is derived from the power of 10, so it is not a significant digit
The two digits to the right of the decimal are significant
.: pH = 3.60](https://image.slidesharecdn.com/01significantdigits-160404182823/75/01-significant-digits-19-2048.jpg)