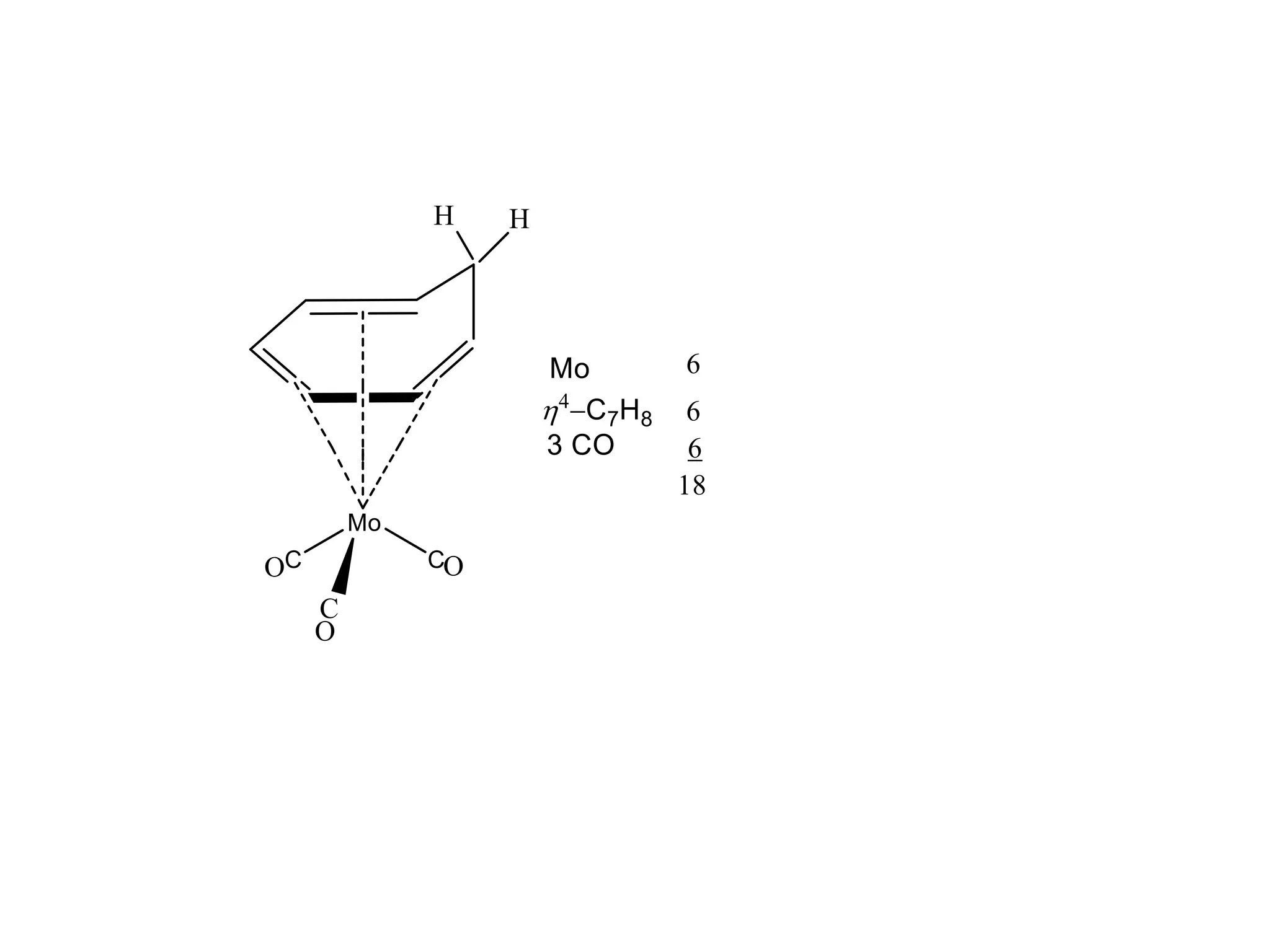
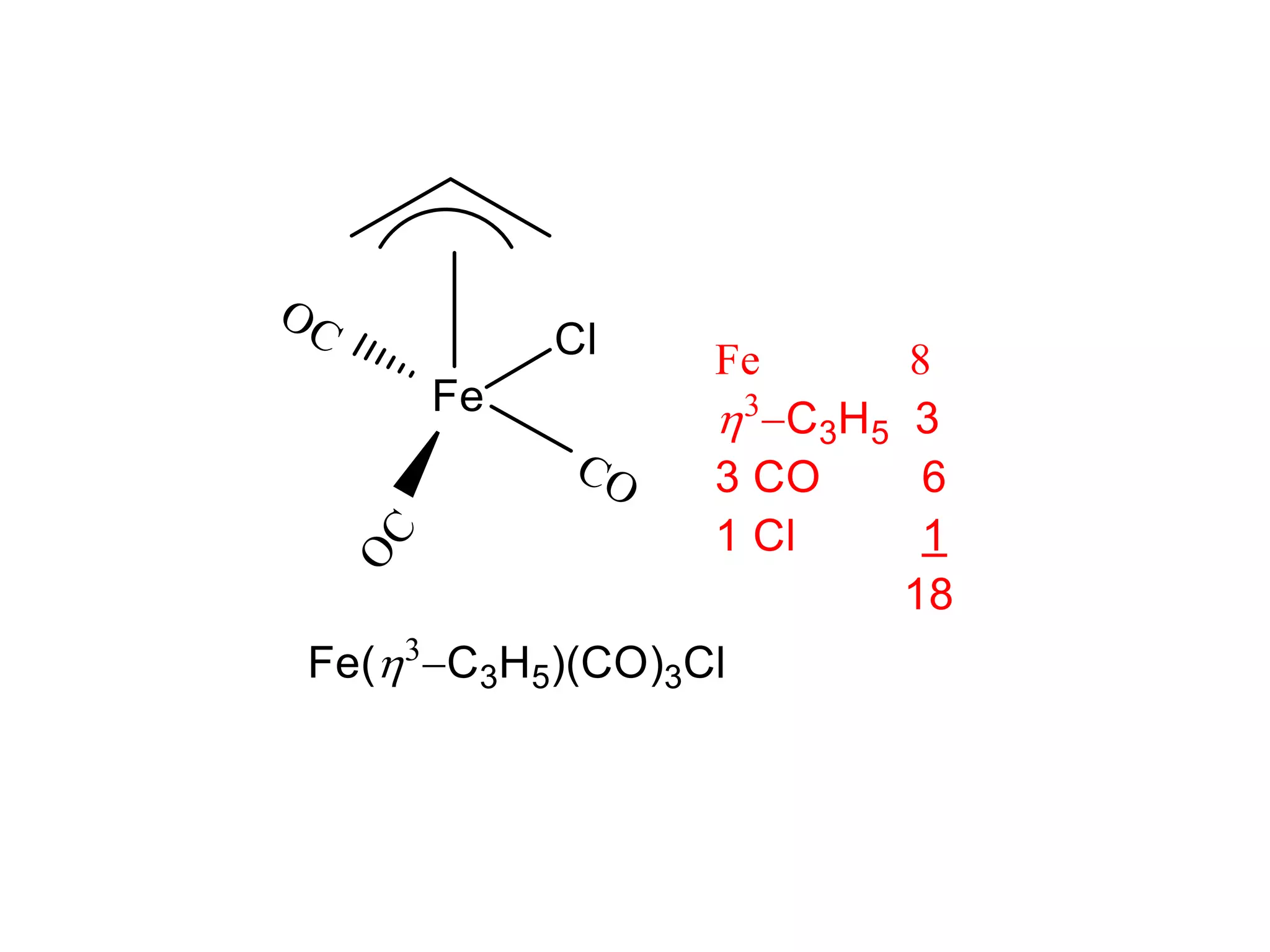
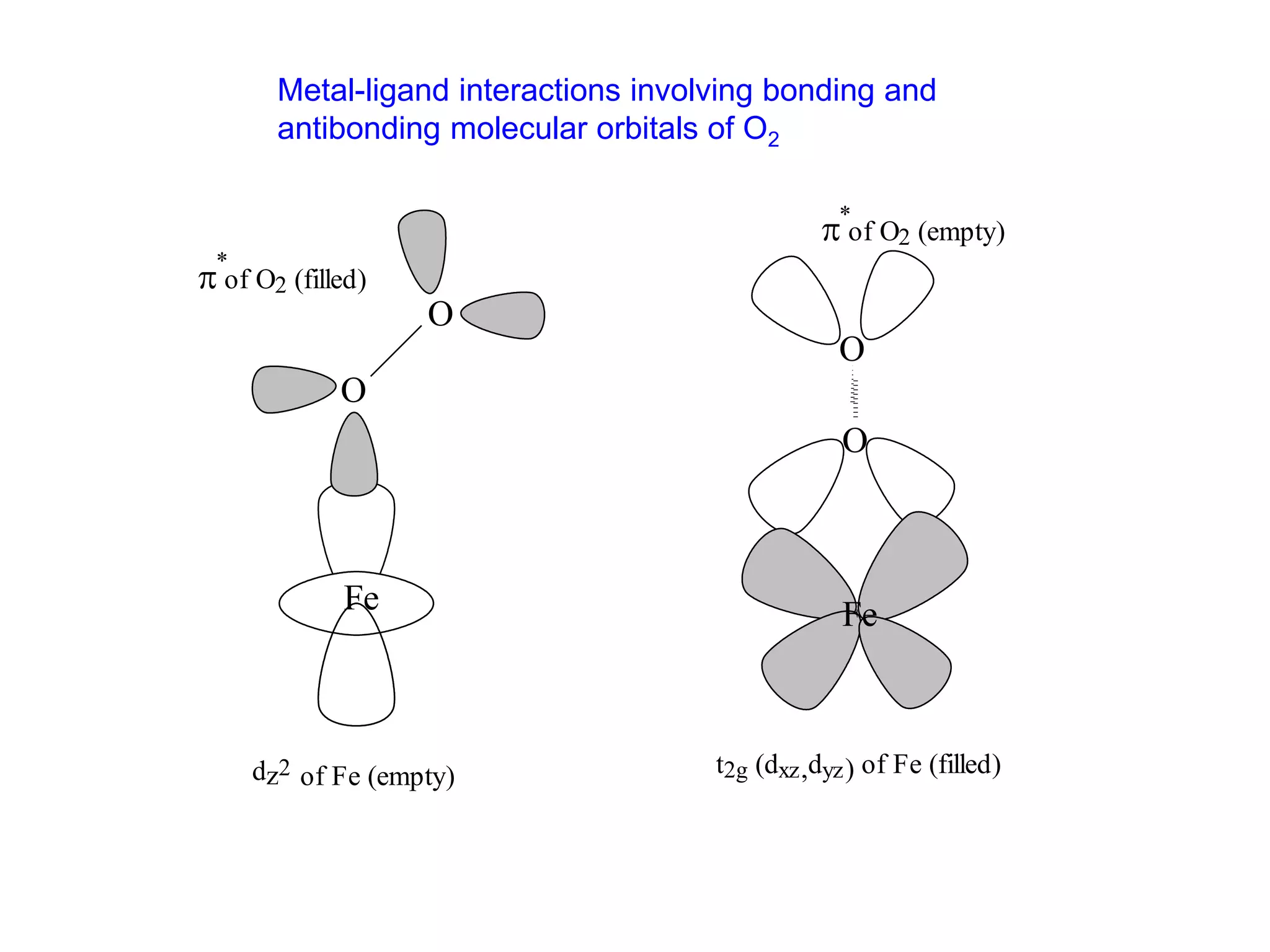
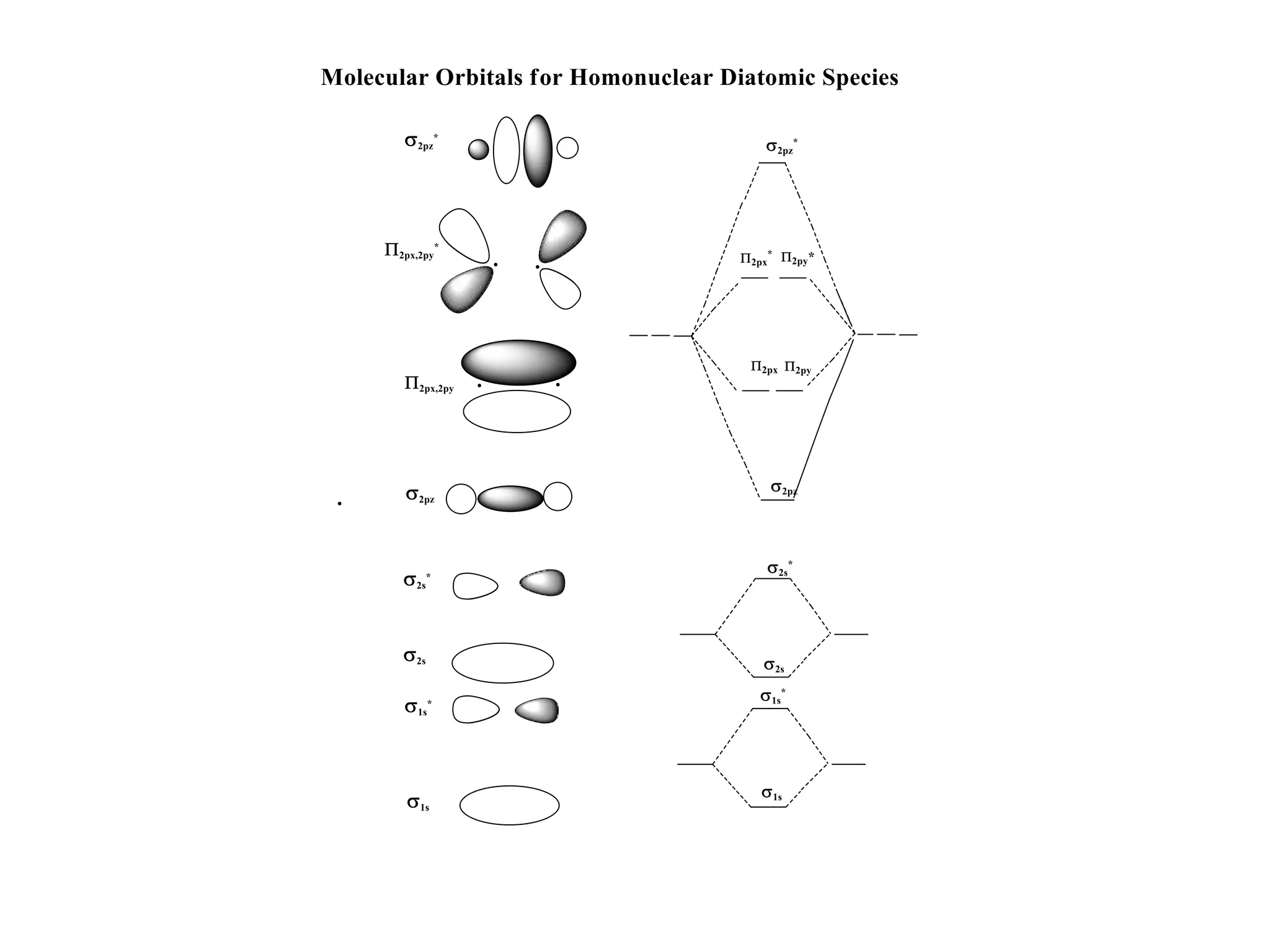
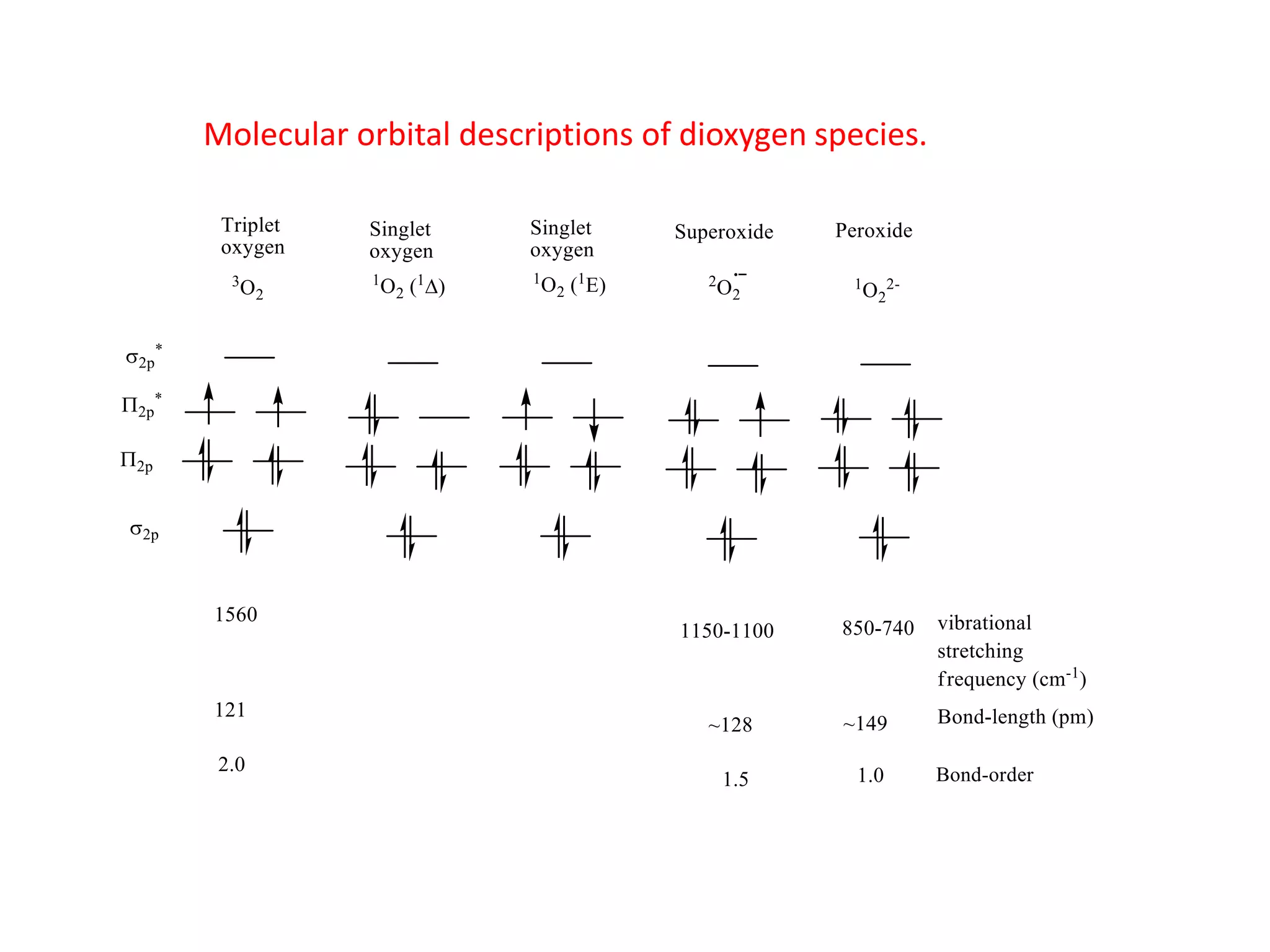
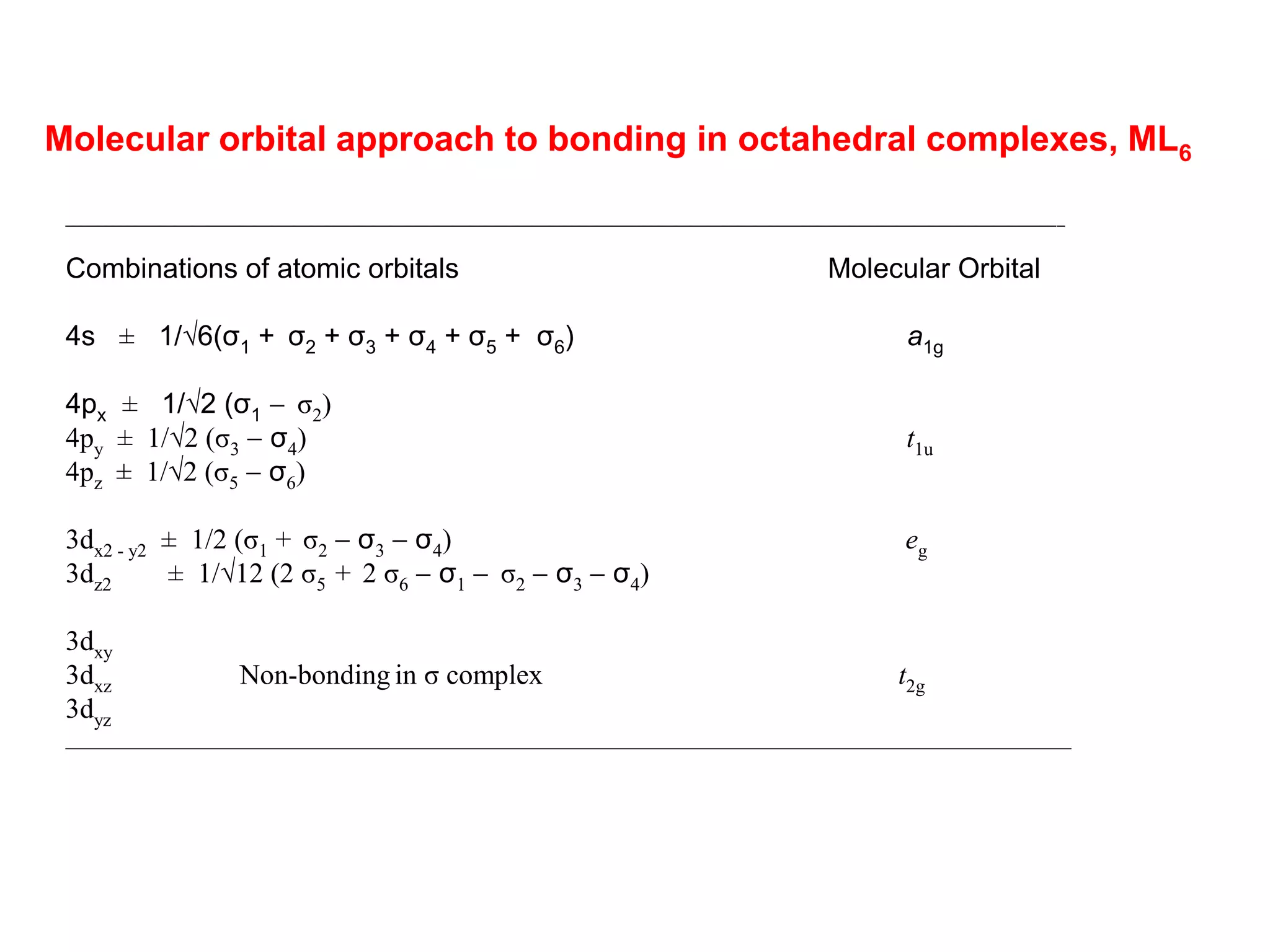
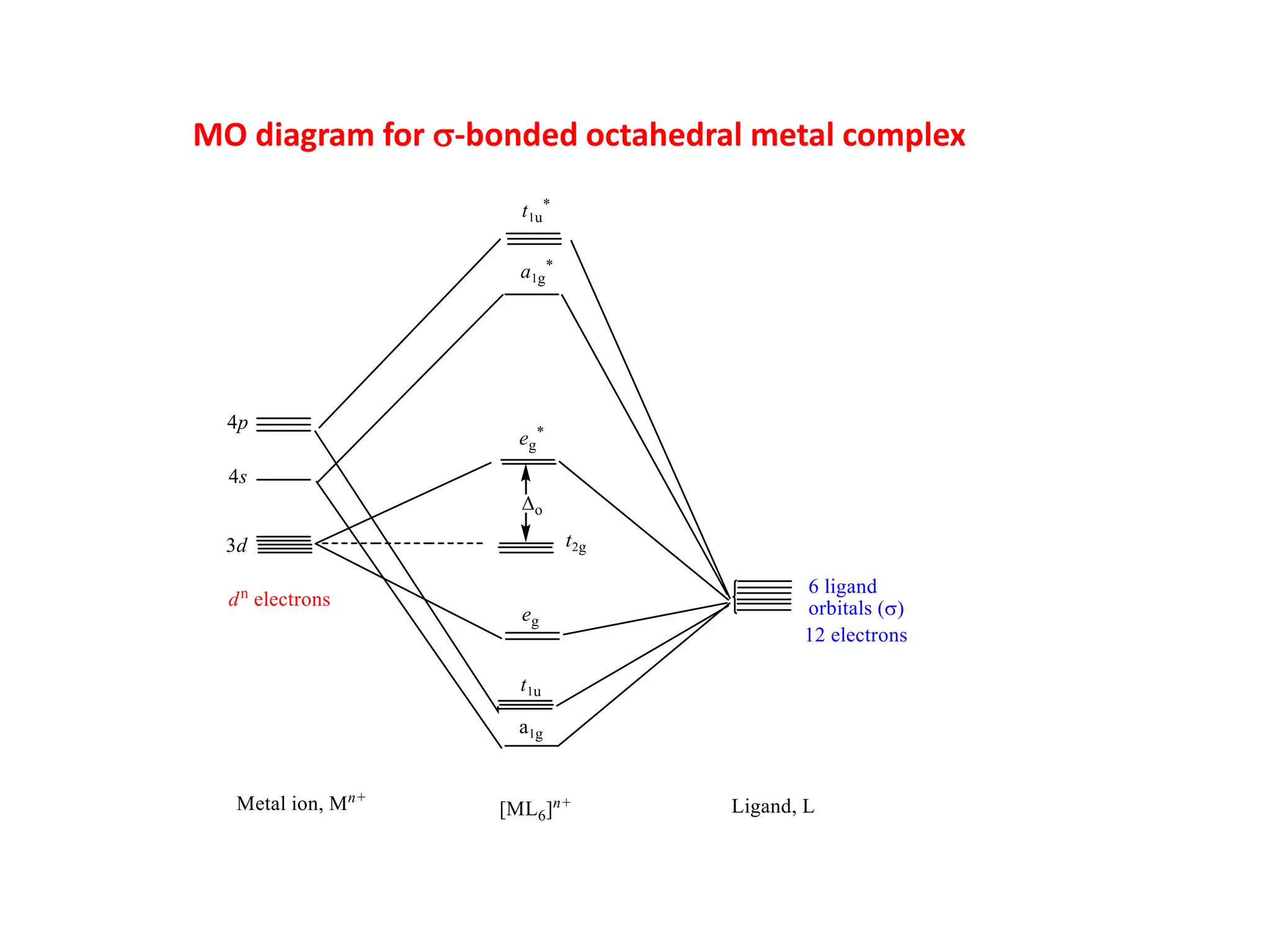
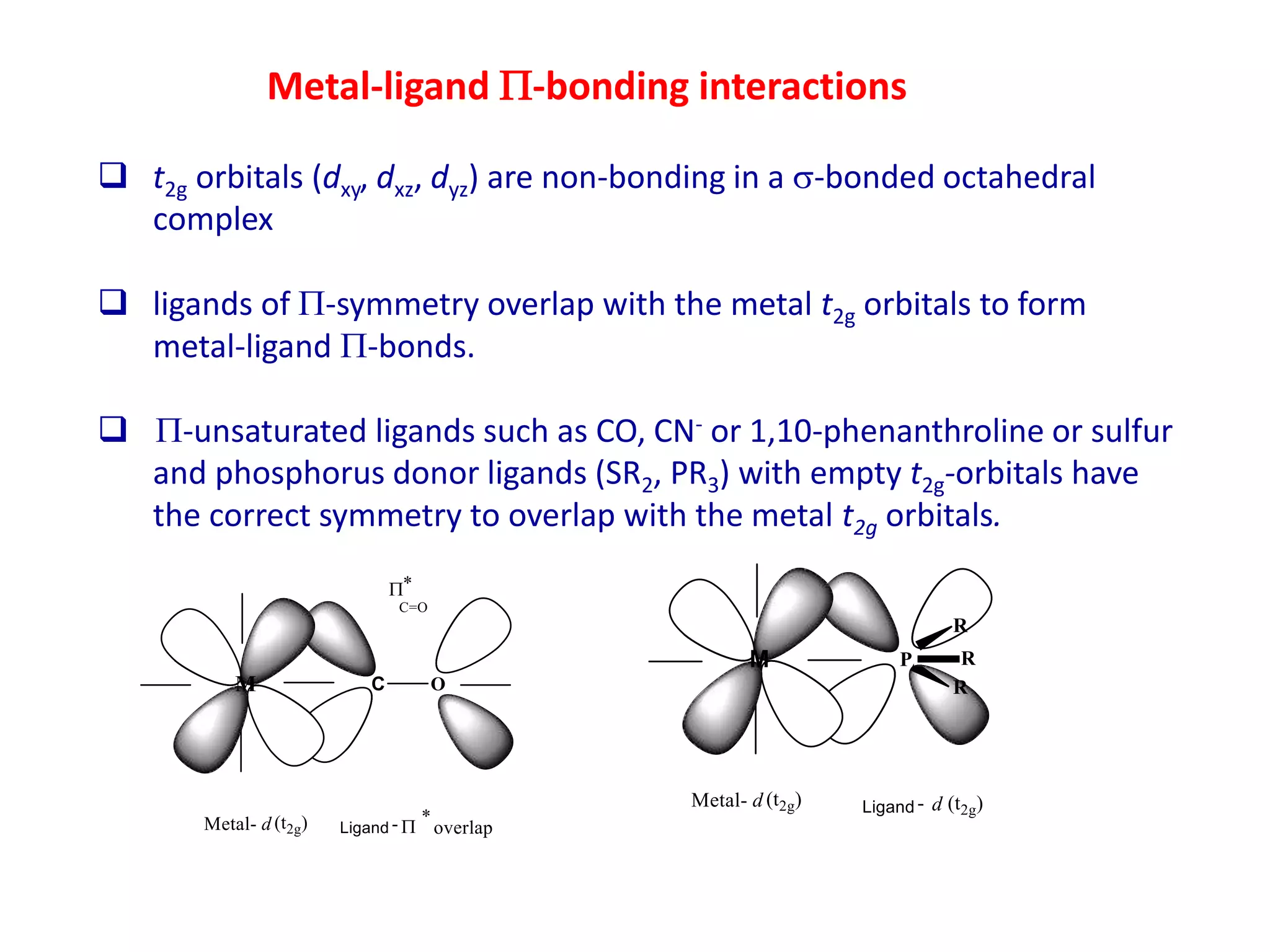
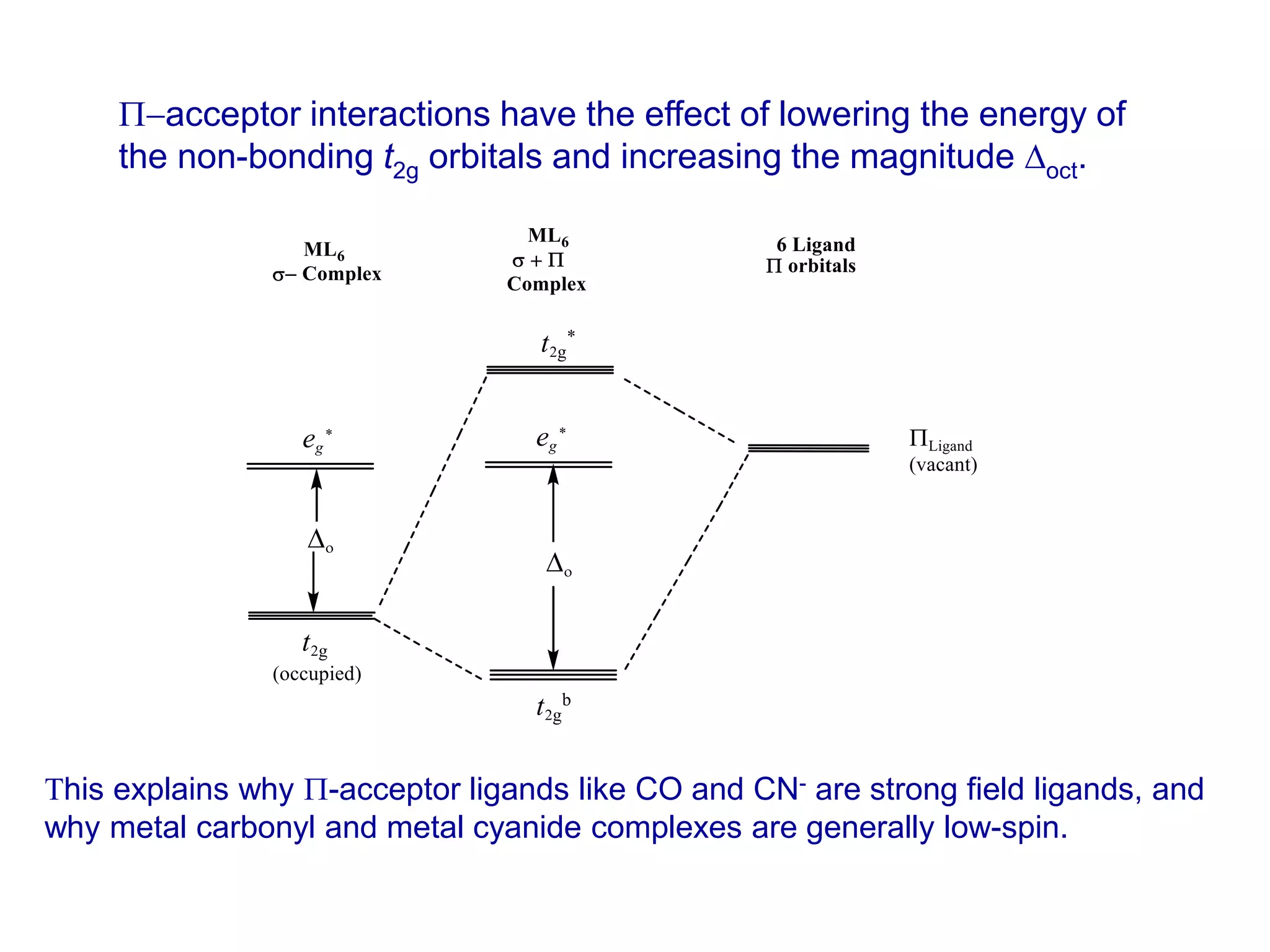
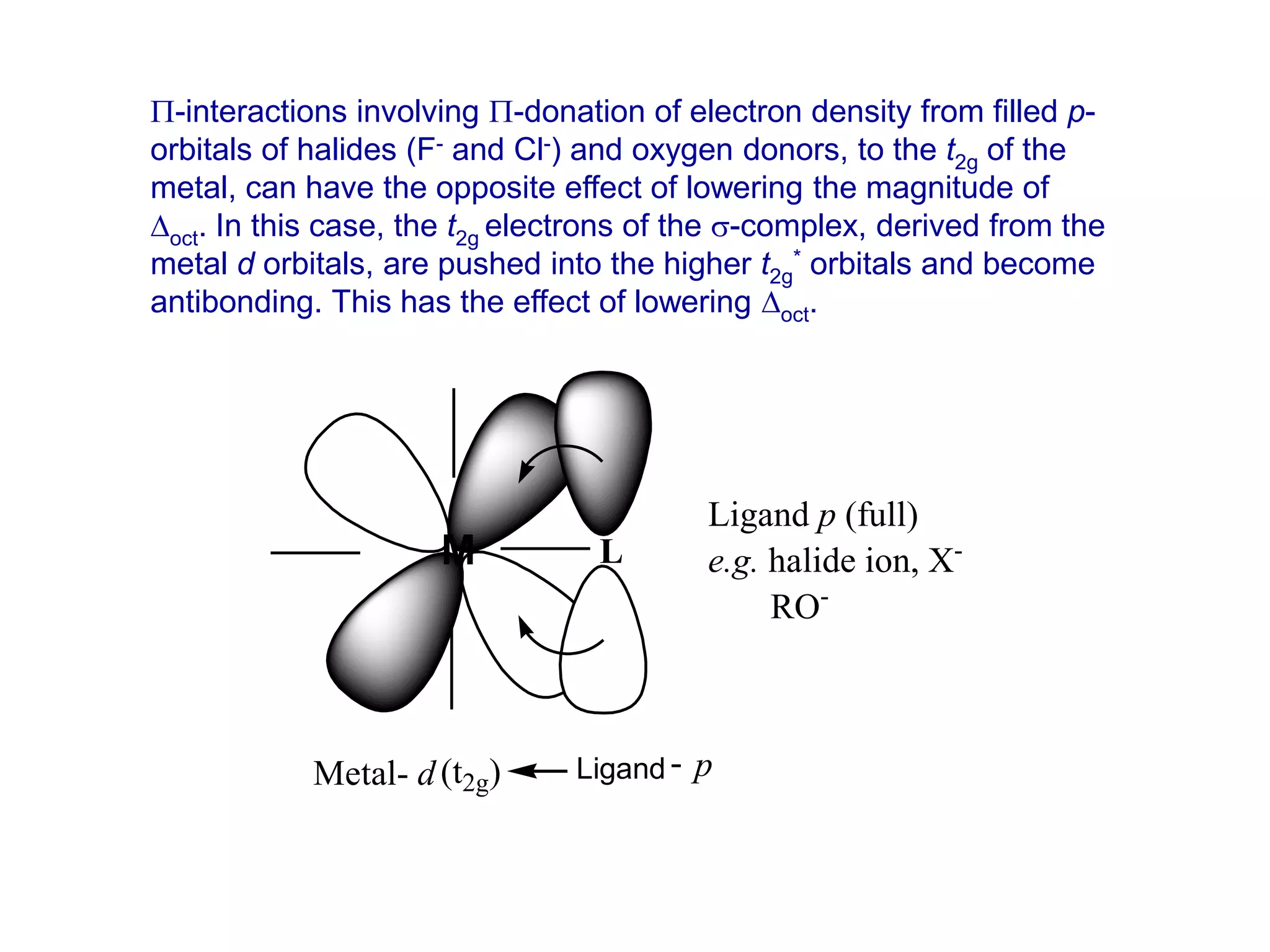
The document discusses molecular orbital theory and its application to transition metal complexes. It describes how atomic orbitals of matching symmetry combine to form molecular orbitals, with equal numbers of bonding and antibonding orbitals. Electrons fill the molecular orbitals starting with the lowest energy orbitals. Ligand interactions such as π-accepting and π-donating affect the splitting of orbitals and influence the metal's oxidation state.




![P-alkene organometallic complexes
Zeise’s Salt, K[PtCl3(C2H4)]](https://image.slidesharecdn.com/3-221107133815-c0cd311b/75/3-Molecular-Orbital-Theory-2011-ppt-13-2048.jpg)