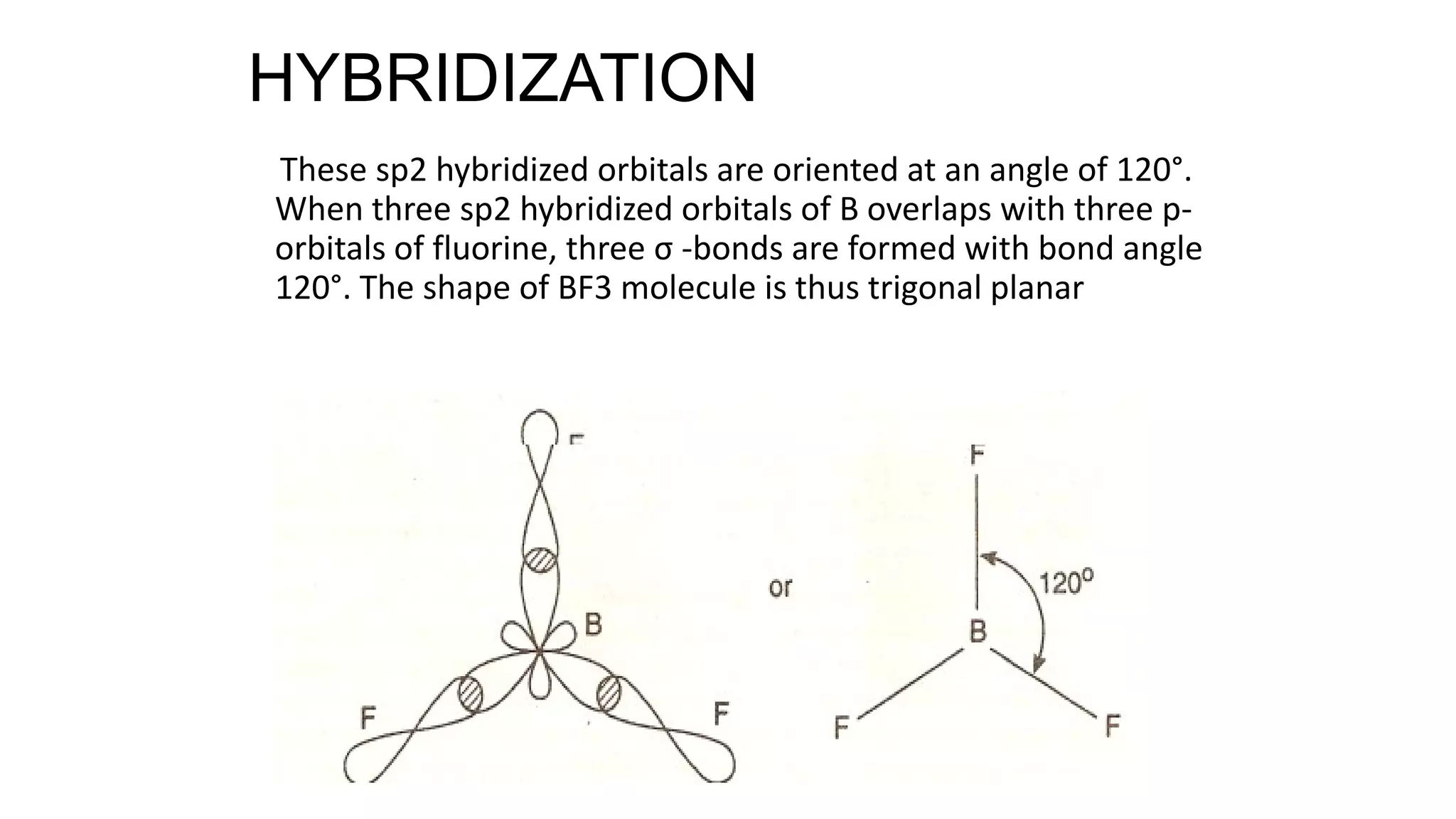
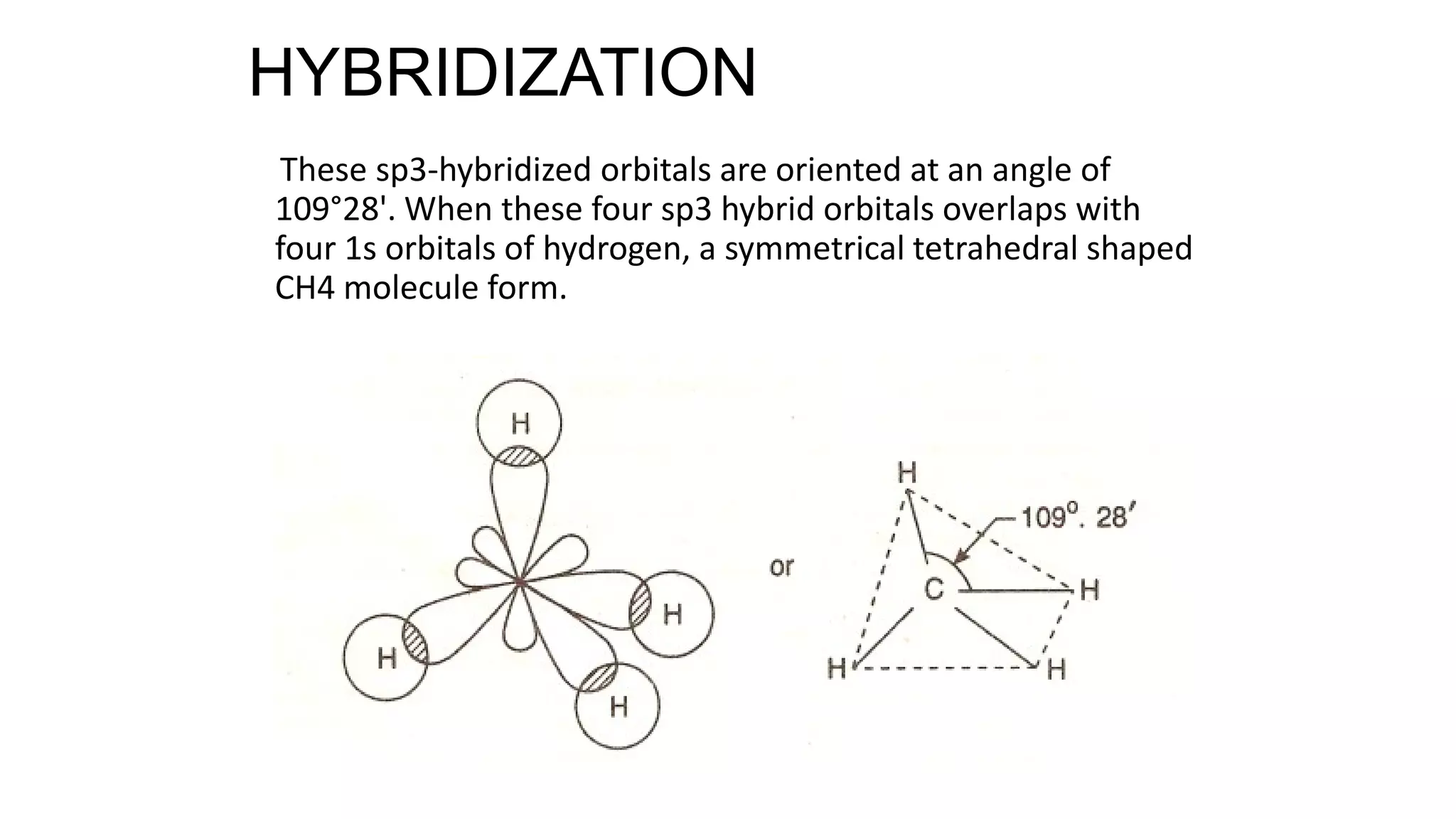
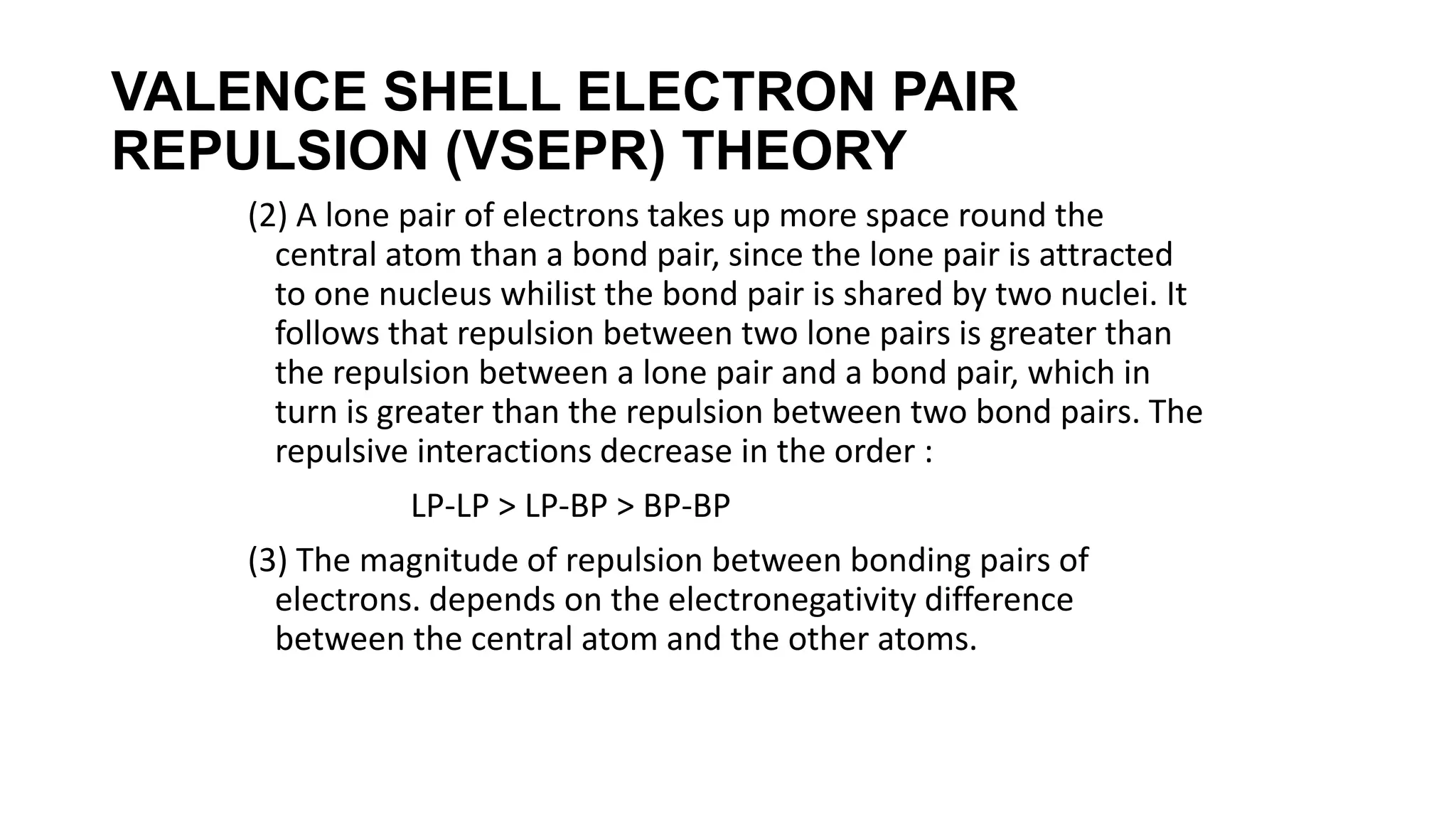
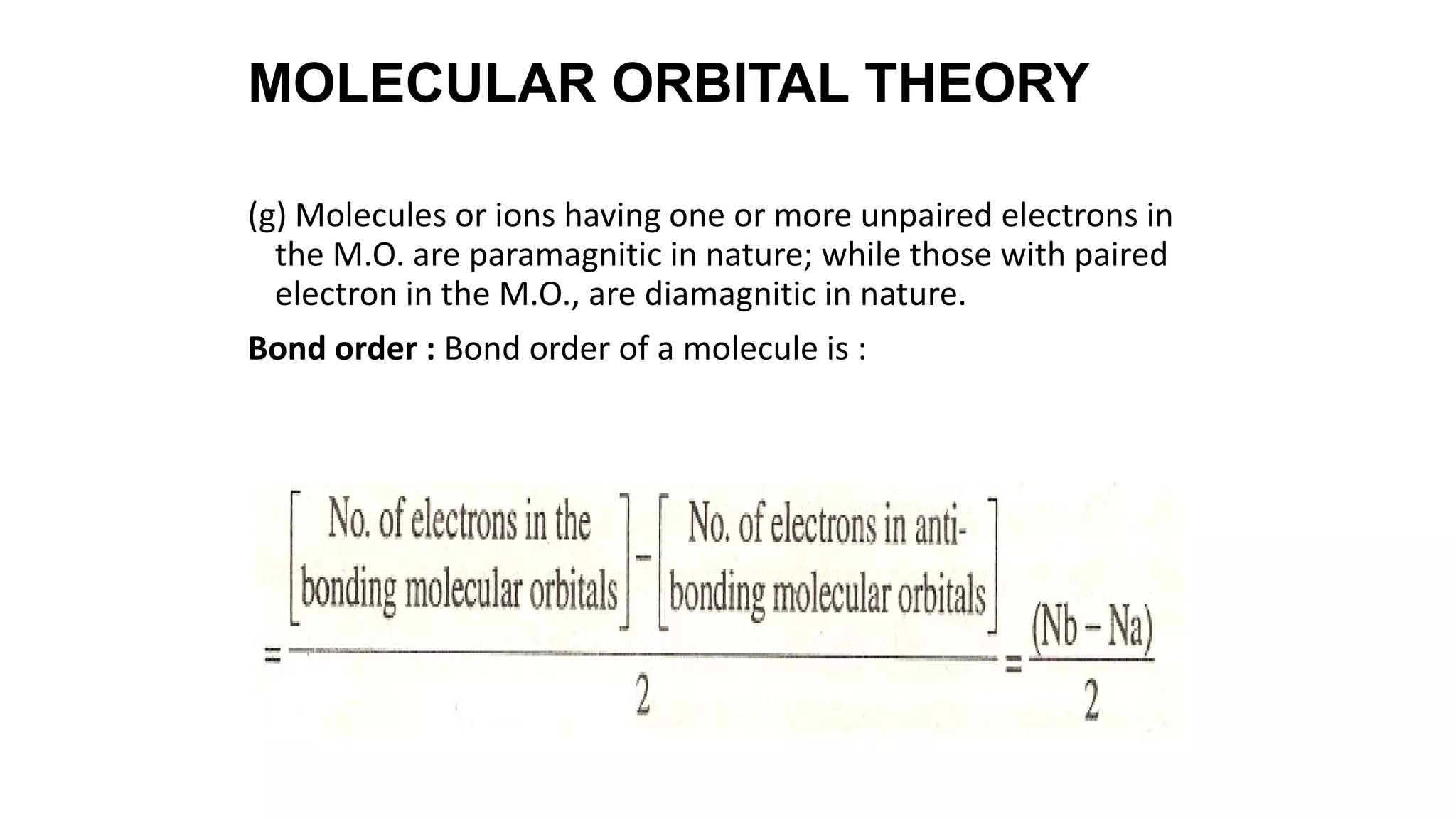
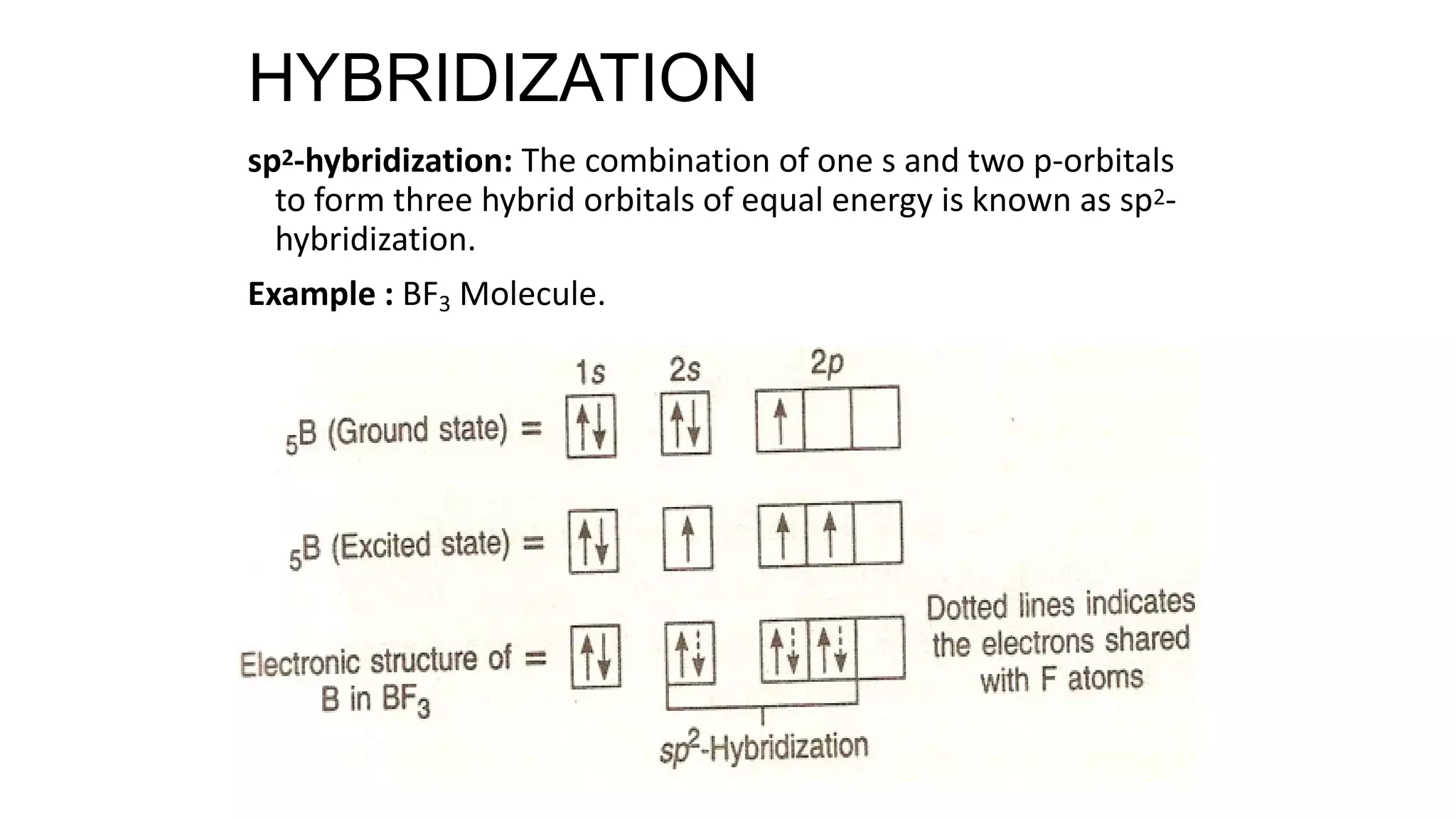
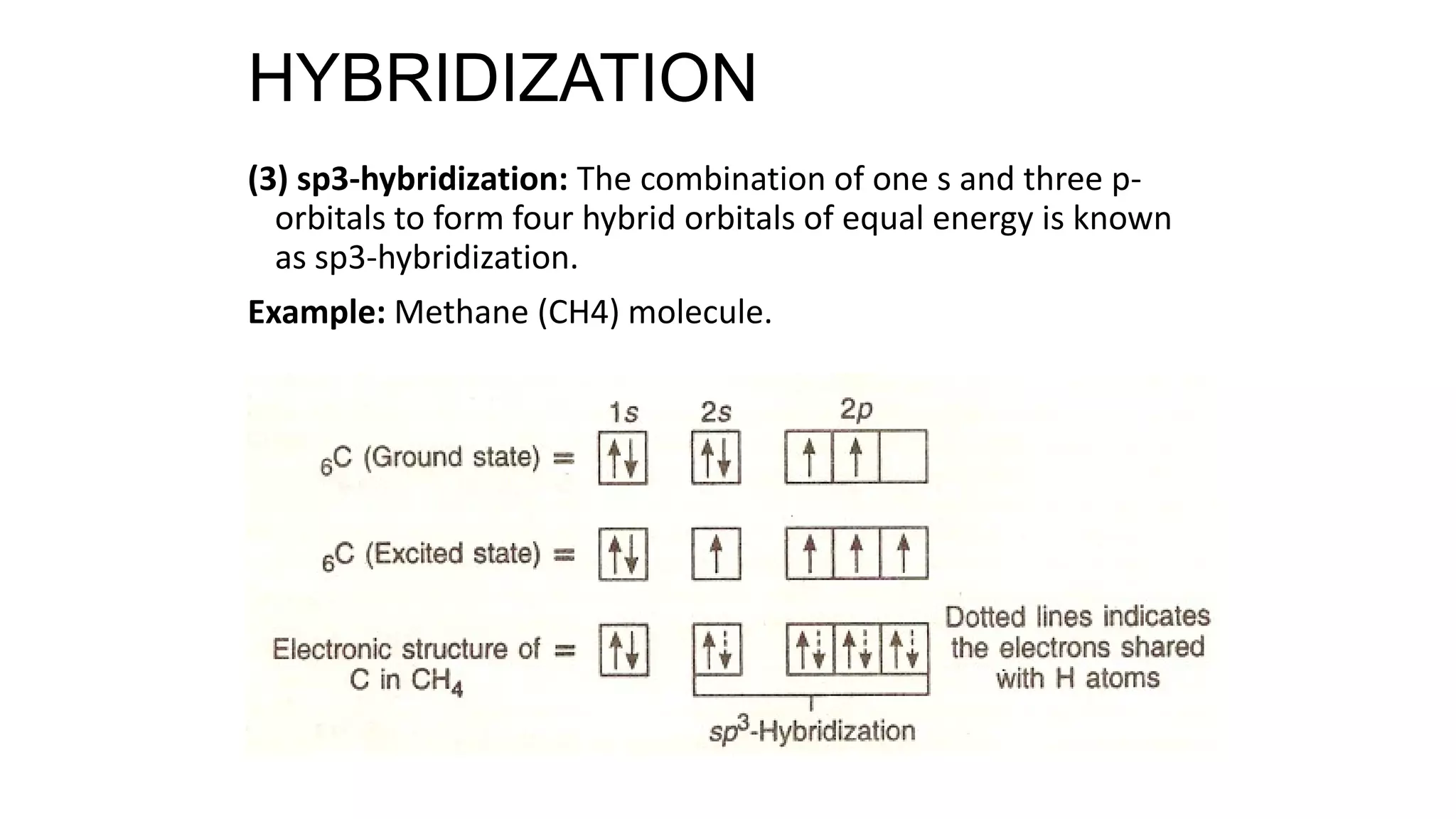
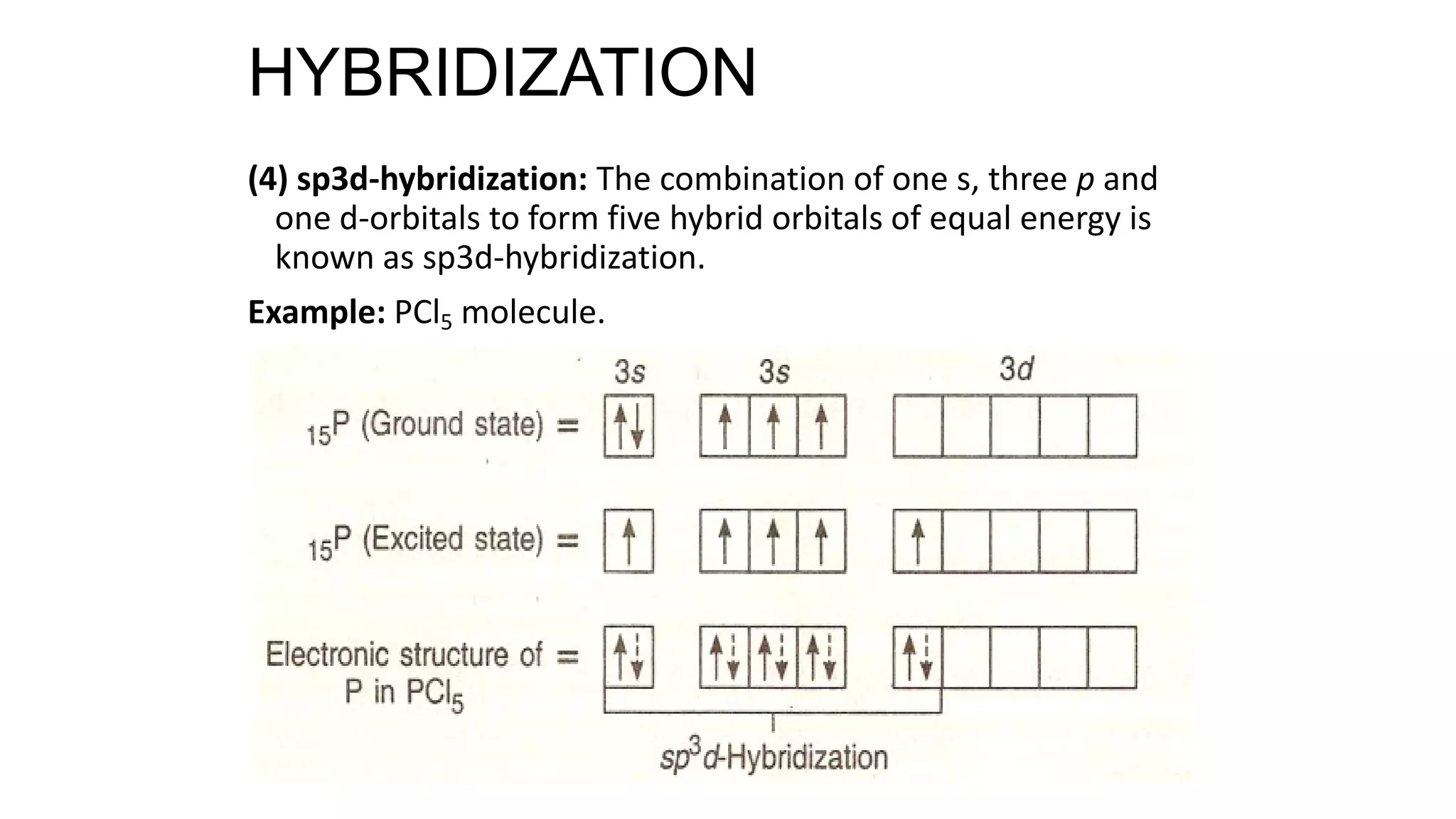
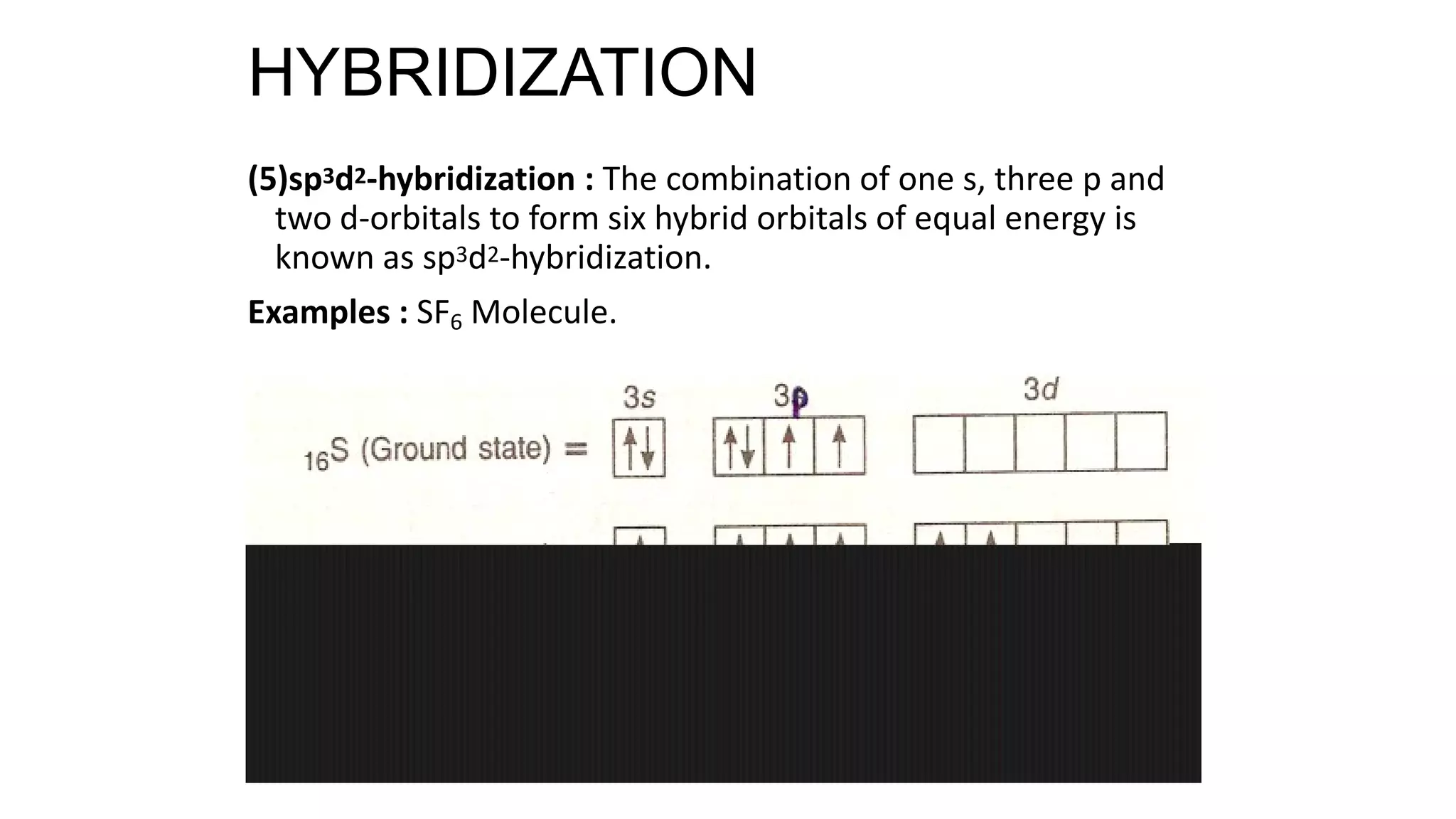
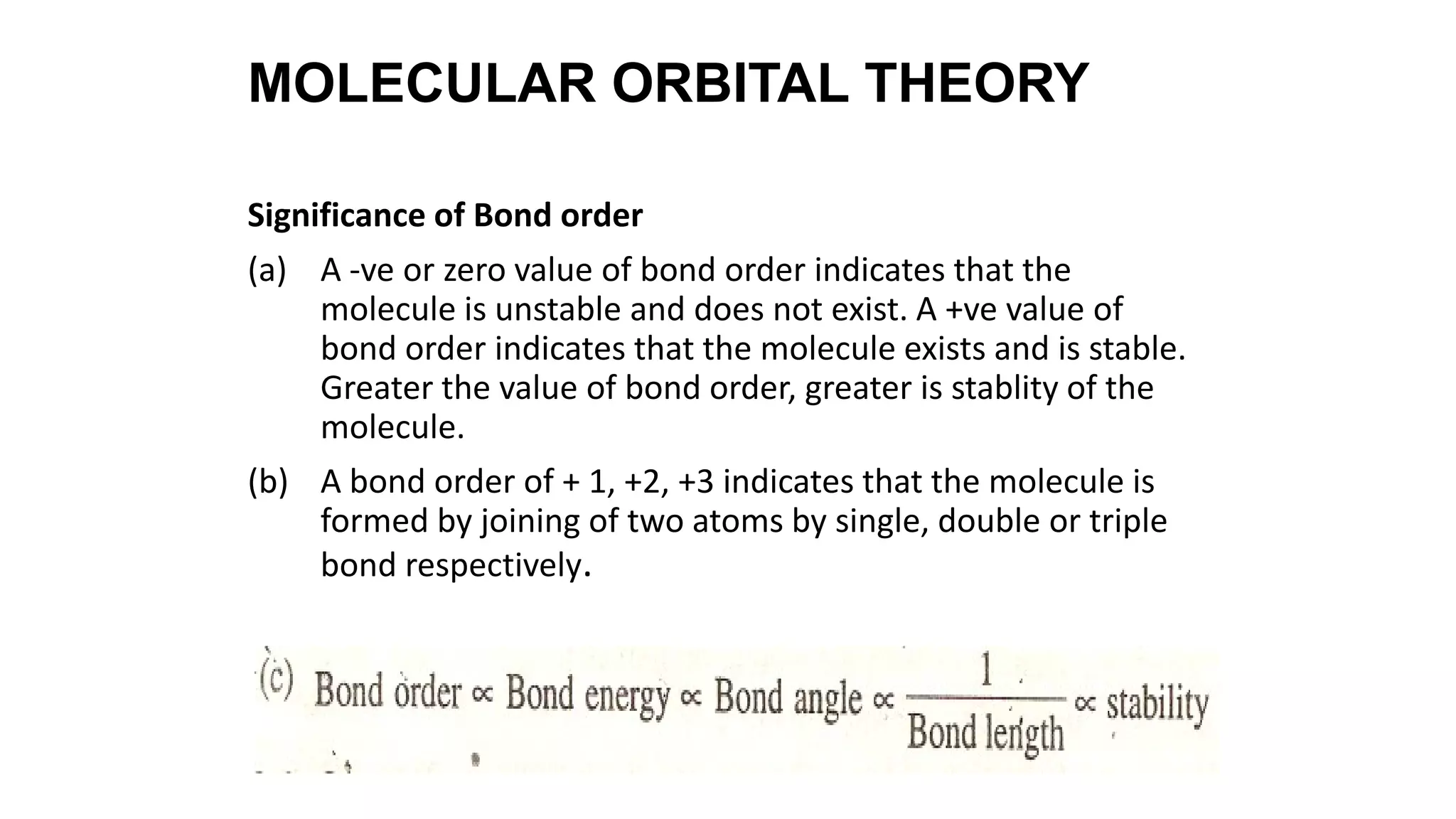
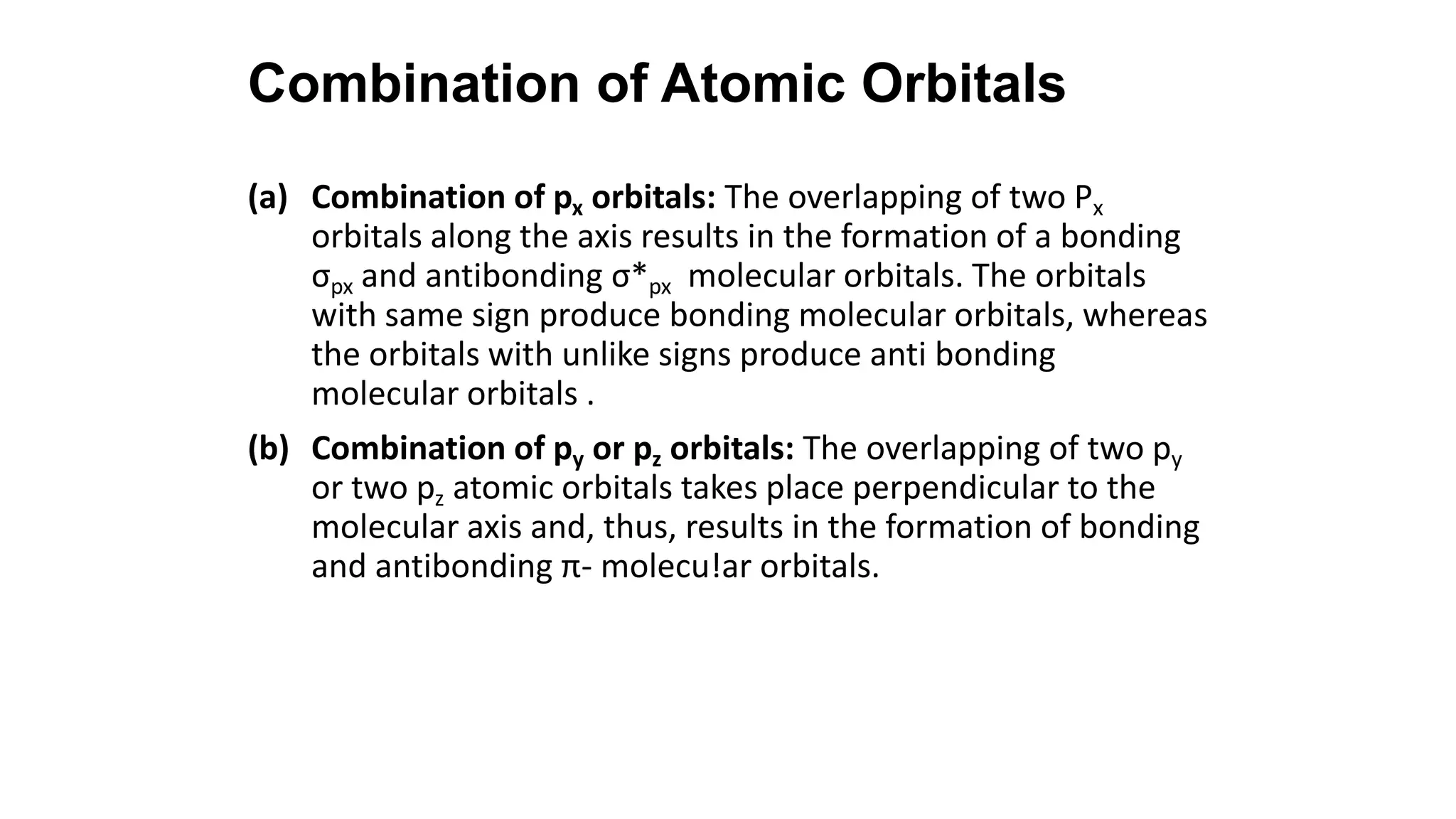
Hybridization involves the mixing of atomic orbitals of similar energies to form new hybrid orbitals for molecule formation. Common hybridizations include sp, sp2, and sp3 which involve mixing one s orbital with varying numbers of p orbitals. The Valence Shell Electron Pair Repulsion (VSEPR) theory states that molecular geometry is determined by repulsions between electron pairs on an atom. Bonding pairs experience less repulsion than lone pairs. Molecular Orbital Theory treats bonding as arising from the combination of atomic orbitals into new molecular orbitals. Bond order corresponds to the number of bonds between atoms.