1. The document discusses orbital hybridization and bonding in methane, ethane, ethylene, and acetylene. It explains how orbital hybridization of the carbon atoms' orbitals allows them to form the observed bonding arrangements in a way consistent with electron configuration.
2. Specifically, it describes how sp3 hybridization of carbon in methane results in four equivalent orbitals that form tetrahedral bonding. Sp3 hybridization is also used to explain the bonding in ethane.
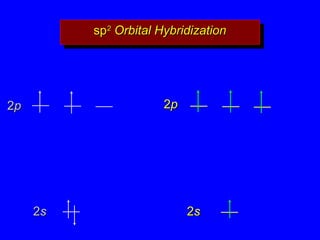
3. Sp2 hybridization is used to explain the planar structure and bonding of ethylene, including the σ and π bonds of the carbon-carbon double bond.
4. Acetylene is described using sp