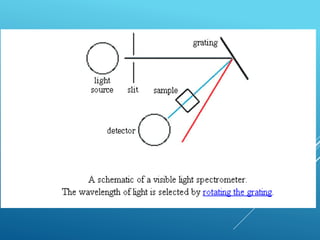
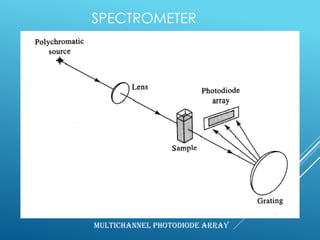
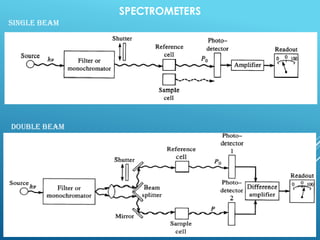
A spectrophotometer uses monochromatic light to measure the absorbance of light by a sample, allowing identification and quantification of compounds. It works by passing light through a sample and measuring the intensity of transmitted light, using this to determine concentration according to the Beer-Lambert law. Key components include a light source, monochromator, sample cuvettes, and detectors, and it has various applications in qualitative and quantitative analysis of proteins, nucleic acids, and other biological compounds.