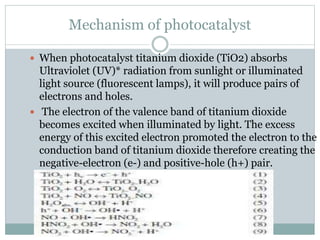
TiO2 is a commonly used photocatalyst that uses light energy to accelerate chemical reactions without being consumed in the process. Dr. Fujishima discovered in the 1960s that titanium dioxide could split water into oxygen and hydrogen gases when irradiated by light. One property of photocatalytic TiO2 is that it can breakdown organic pollutants like oil, exhaust fumes, and mold using energy from UV light by generating strong oxidizing hydroxyl radicals. While TiO2 is effective due to properties like stability, low toxicity and cost, its large bandgap only activates with UV light which represents only 5% of sunlight, so methods to extend its activity into visible light are being explored.