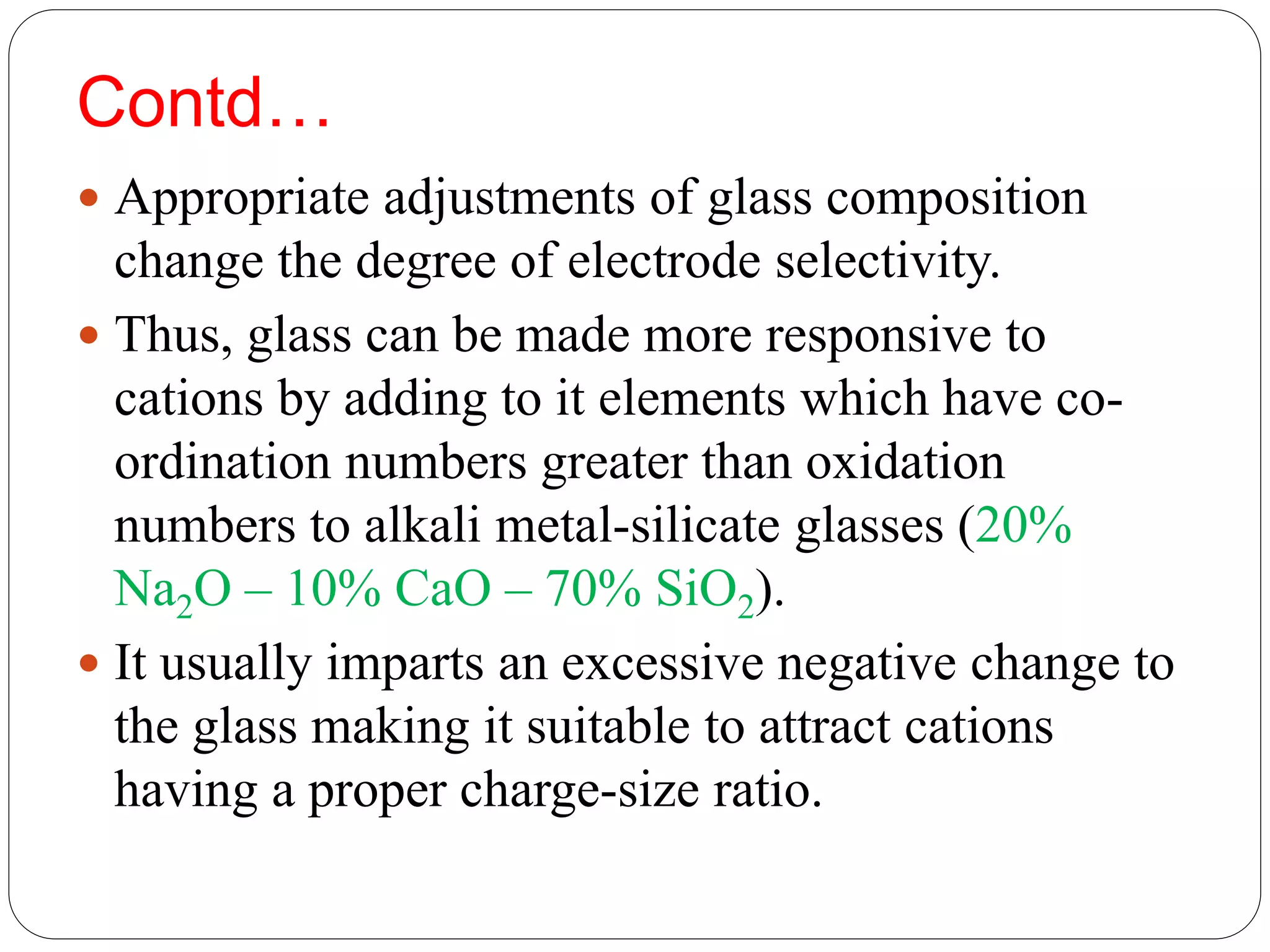
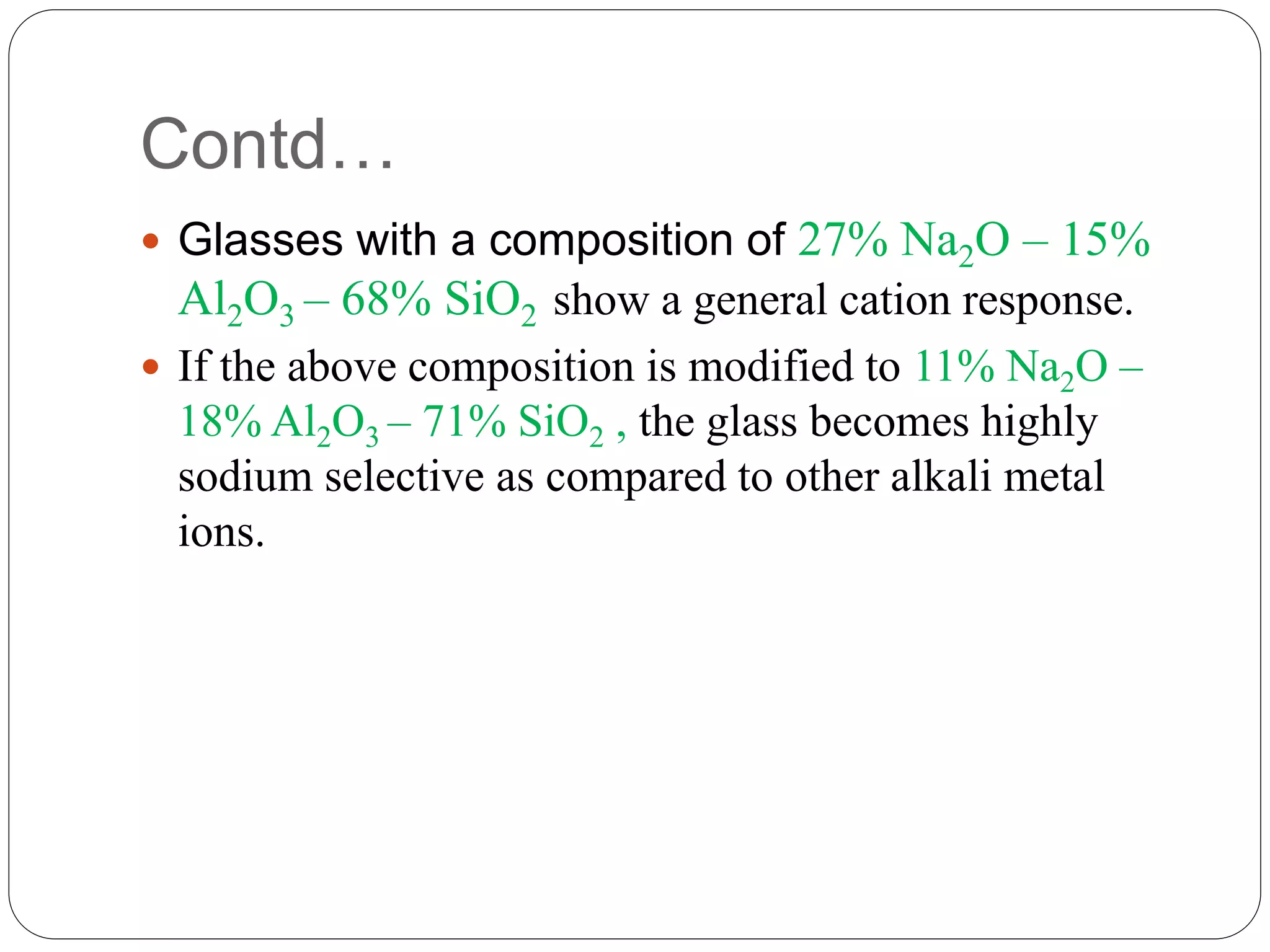
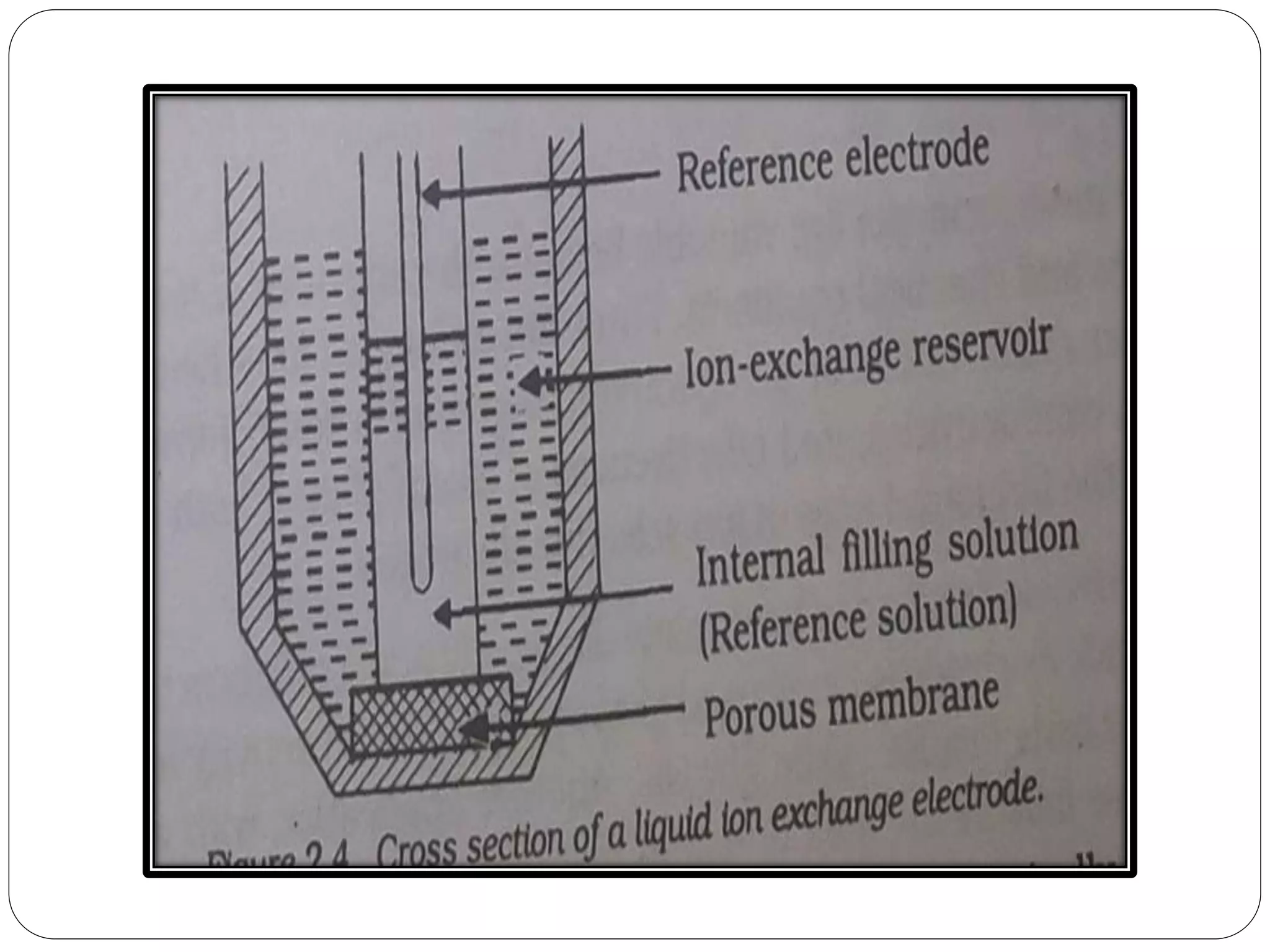
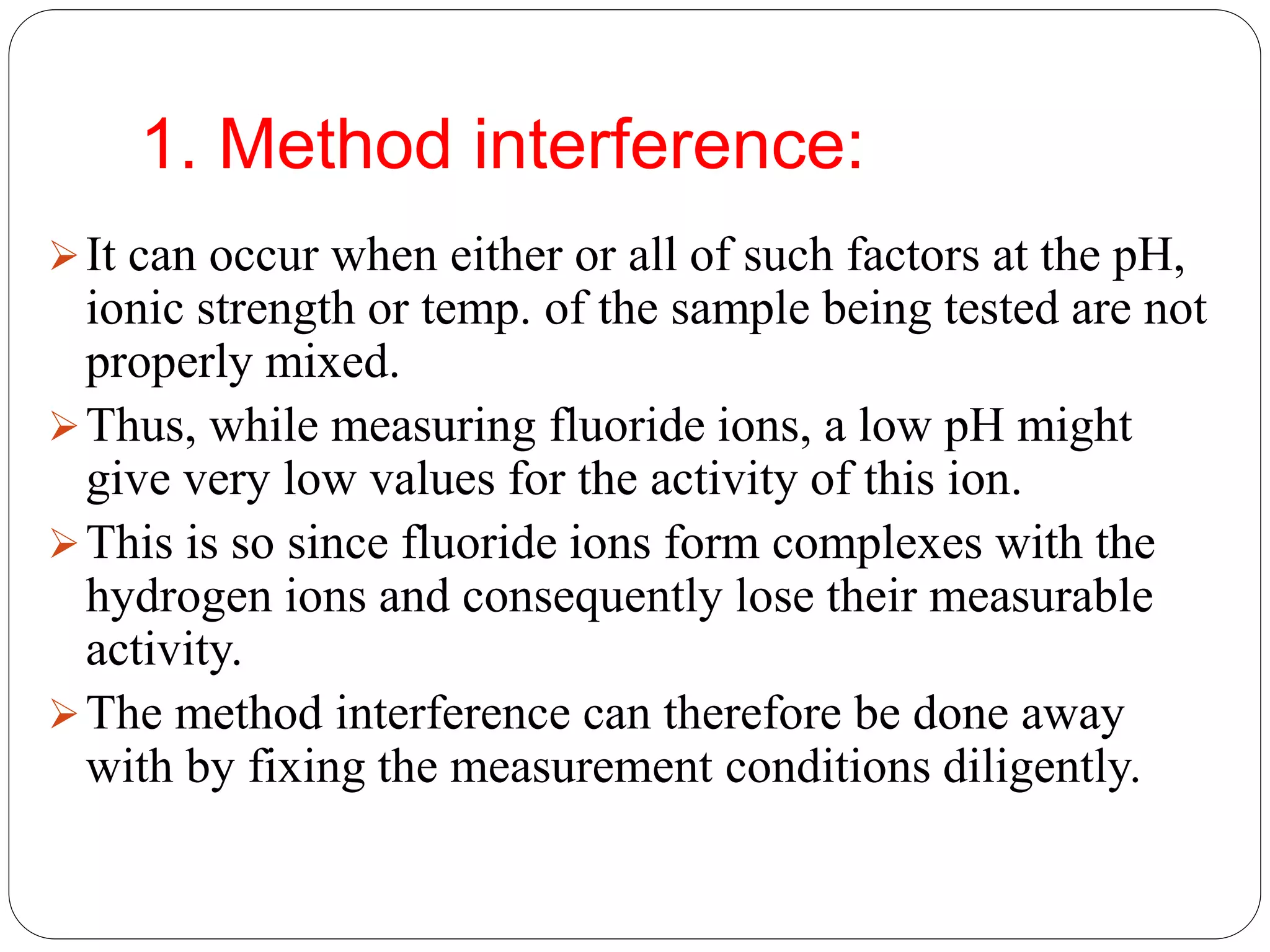
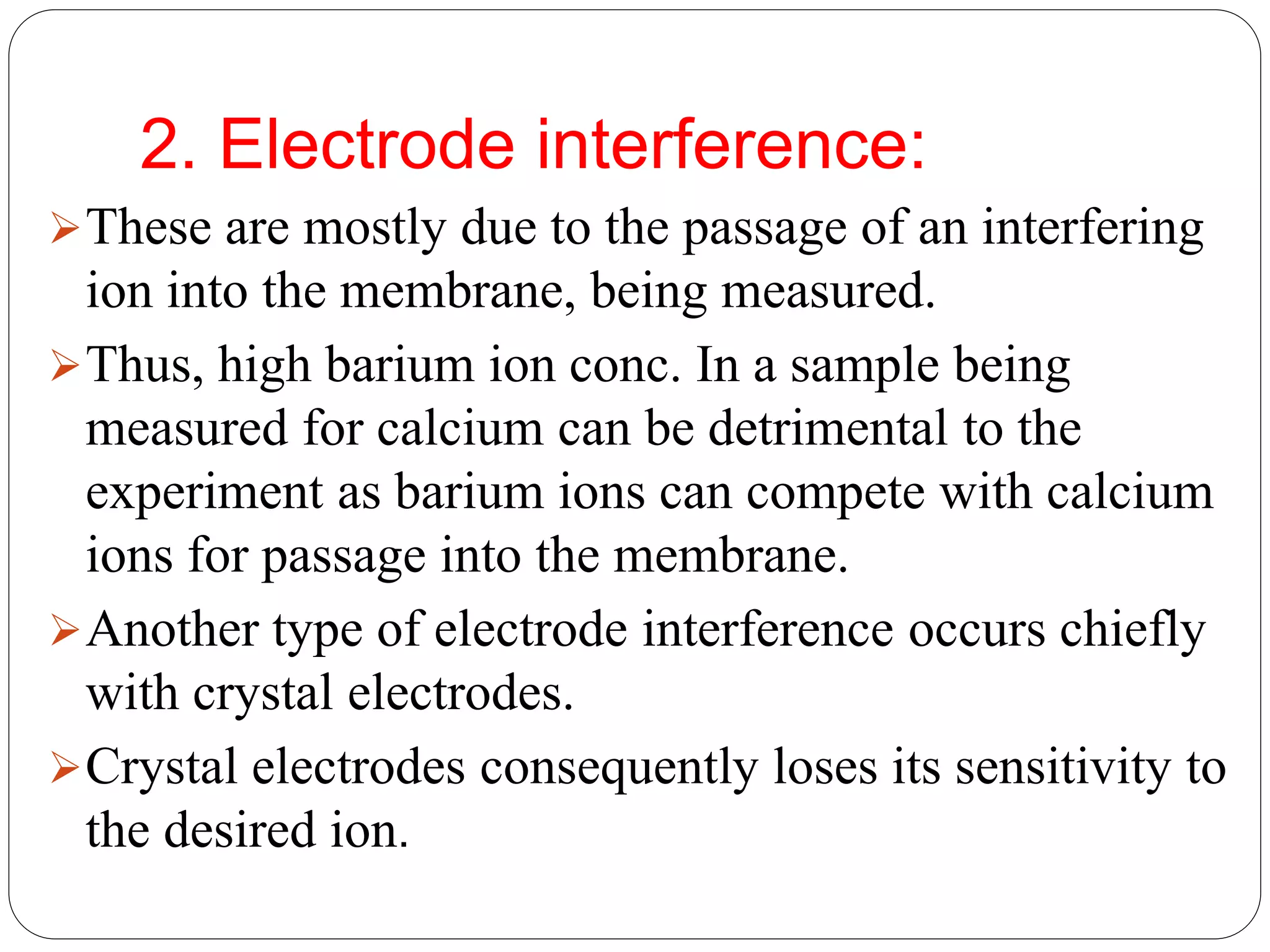
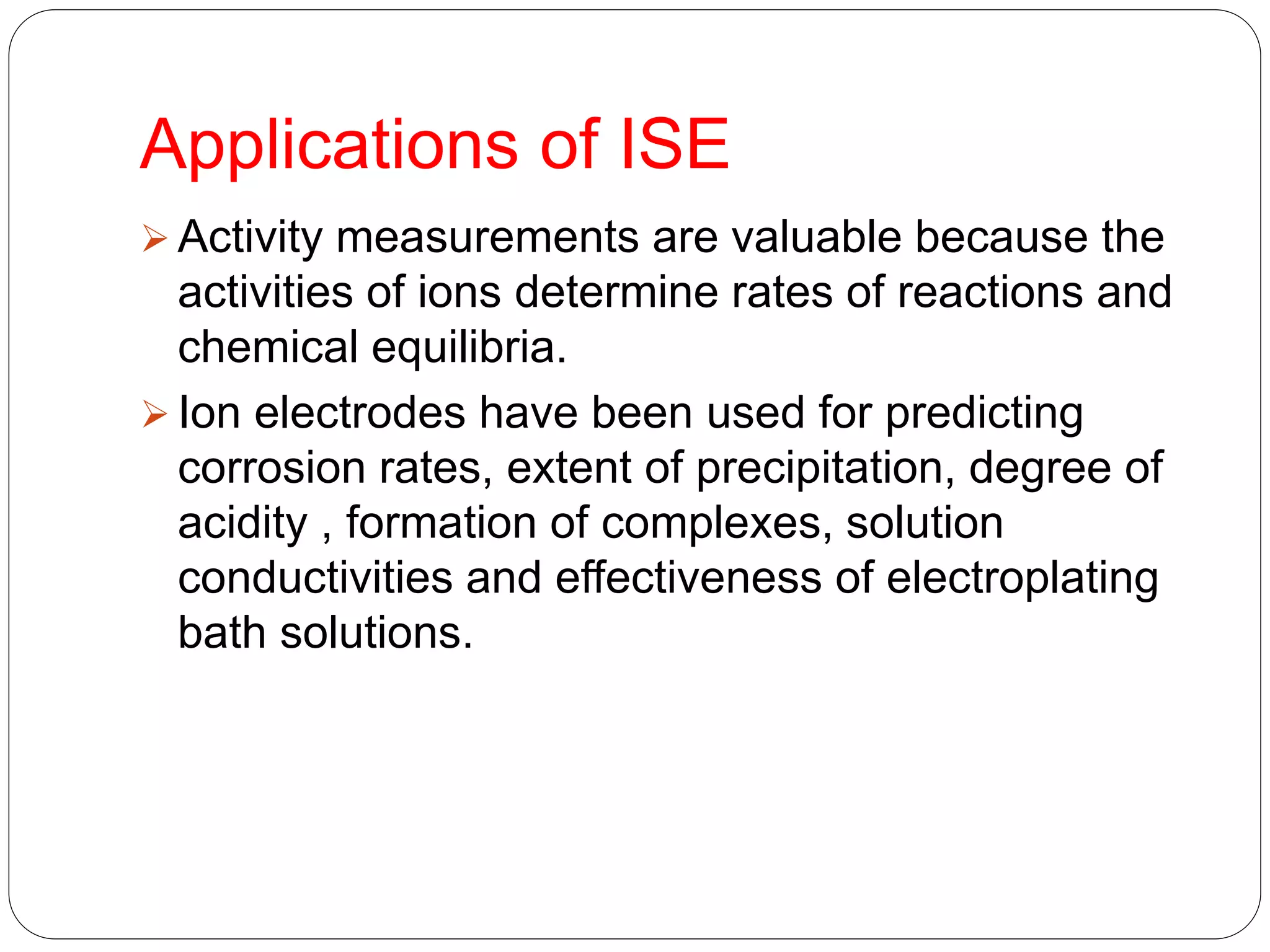
The document discusses ion-selective electrodes (ISEs), highlighting their crucial role in measuring metal ions that affect cellular processes and metabolism. It describes various types of ISEs, including glass membrane, solid-state ion exchange, solid-state crystal, and liquid-membrane electrodes, and outlines their applications in diagnostics and chemical analysis. Furthermore, it addresses interferences and advantages of ISEs, emphasizing their wide response range and low cost.