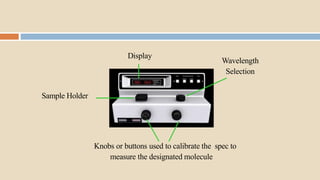
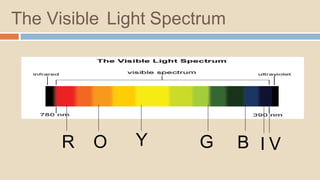
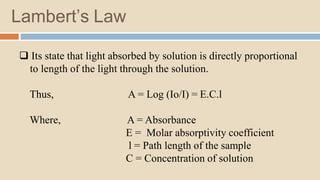
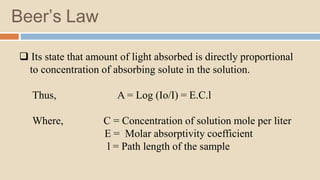
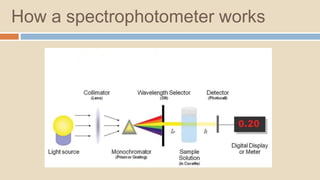
A spectrophotometer is an instrument that measures the amount of light transmitted through a sample. It uses monochromatic light of specific wavelengths, typically in the ultraviolet-visible spectral region. The amount of light absorbed is quantified using the Beer-Lambert law, where absorbance is directly proportional to concentration and path length. There are two main types - UV-visible spectrophotometers use light from 200-700 nm, while visible spectrophotometers use visible light from 350-700 nm. A spectrophotometer works by splitting white light into wavelengths, directing the light through a sample, measuring the transmitted light, and calculating absorbance values using Beer's law. Common applications include determining sample concentration, purity,