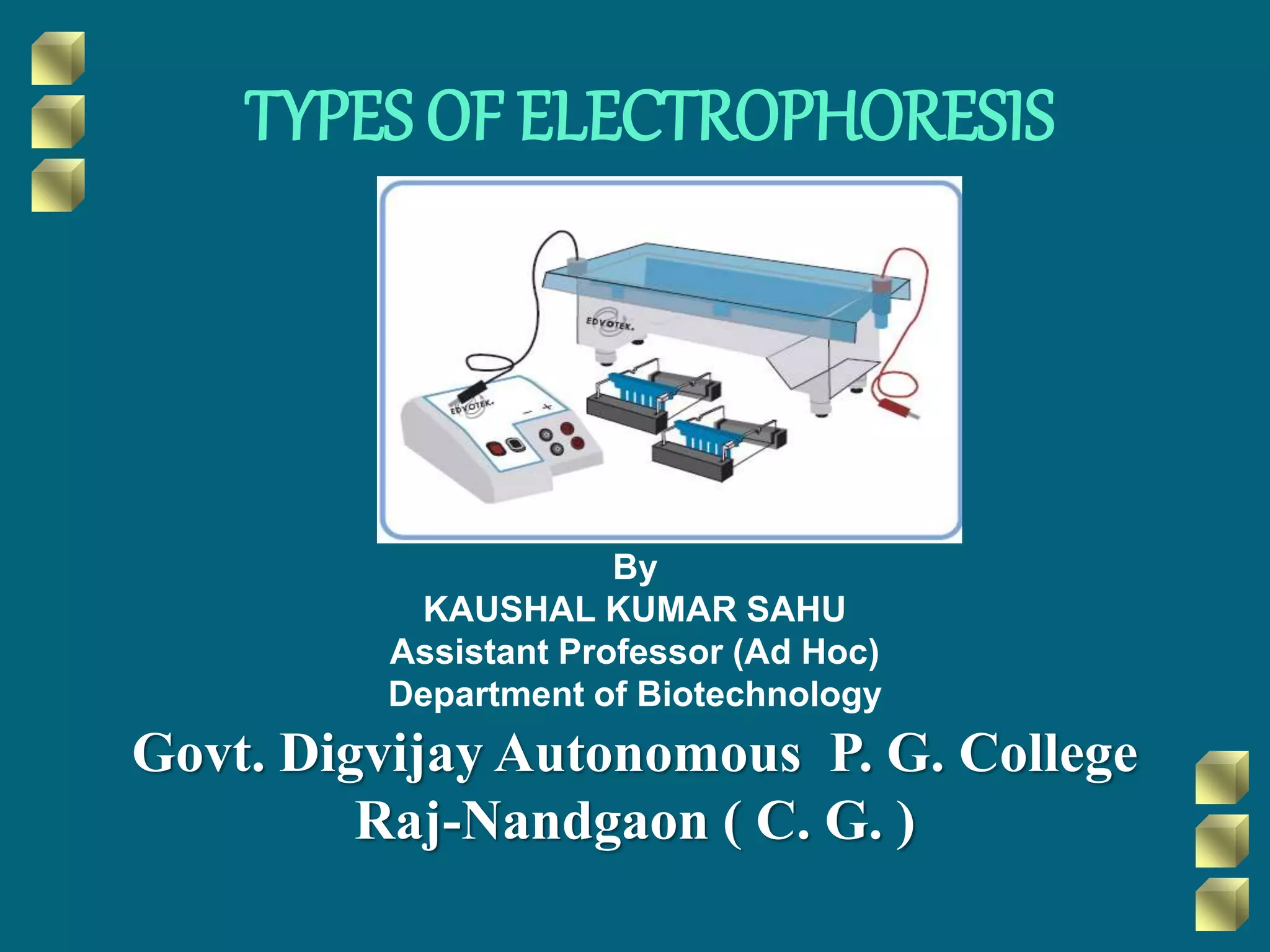
This document discusses different types of electrophoresis techniques. It begins with an introduction to electrophoresis and its history. It then describes various electrophoresis techniques including gel electrophoresis using agarose gel or polyacrylamide gel, capillary electrophoresis, isoelectric focusing, two-dimensional electrophoresis, and immuno electrophoresis. The document explains the basic principles of electrophoresis and how it uses an applied electric field to separate biomolecules like proteins, nucleic acids, and carbohydrates based on their charge and size. It concludes with some applications of electrophoresis techniques.