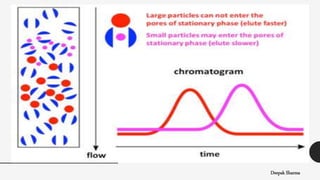
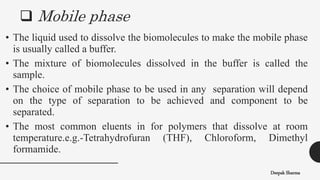
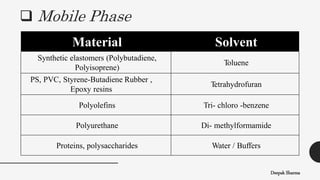
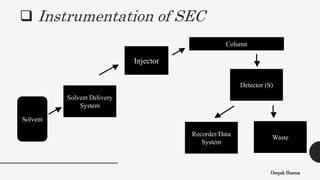
The document is a lecture on size exclusion chromatography (SEC), a method used for separating molecules based on their size, commonly applied to large biomolecules like proteins and synthetic polymers. It outlines the principles, components, instrumentation, advantages, and applications of SEC, emphasizing the importance of selecting appropriate stationary and mobile phases for effective separation. Additionally, it highlights the benefits of SEC, including short analysis times and well-defined separations, while addressing potential disadvantages and specific applications in protein purification and molecular weight determination.