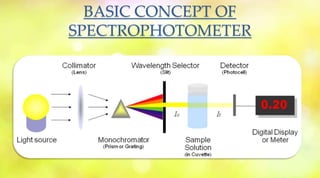
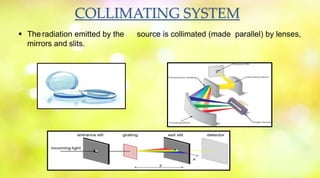
Spectrophotometry uses the absorption of light by chemical substances to measure concentration. A spectrophotometer directs a beam of light through a sample and measures the intensity of transmitted light, relating it to concentration through Beer's Law. It operates based on Lambert's Law stating light absorption increases with concentration and path length. Common types are single and double beam instruments, with the latter measuring sample and reference simultaneously. Components include a light source, monochromator, sample holder, and detector. Applications include quantifying analytes and studying reaction kinetics and molecular structure.