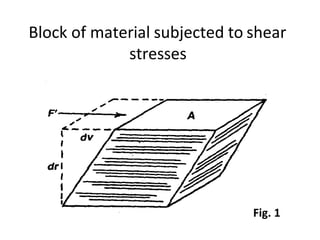
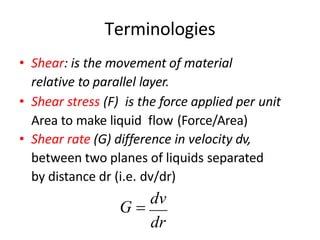
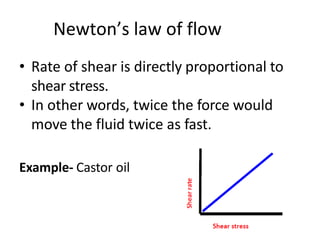
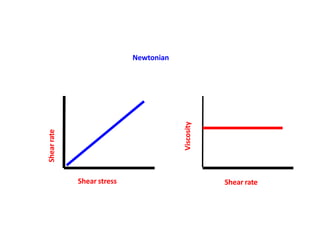
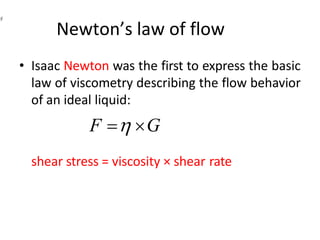
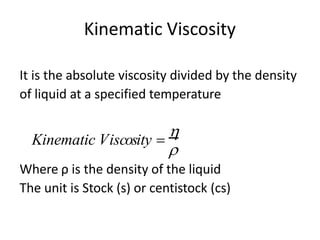
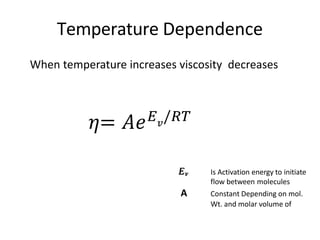
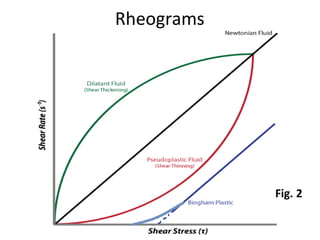
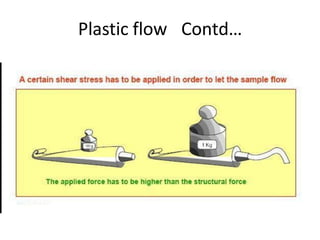
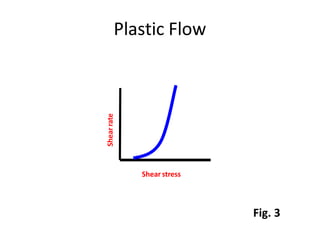
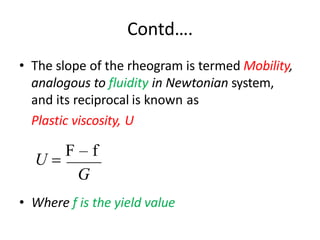
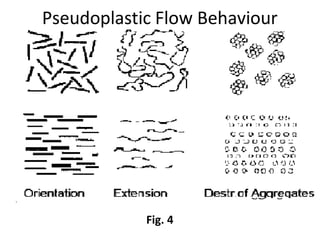
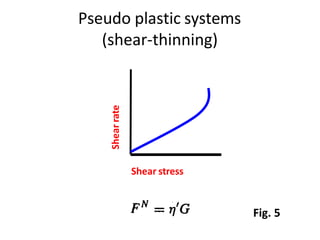
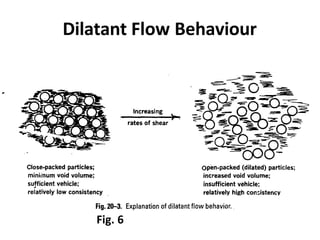
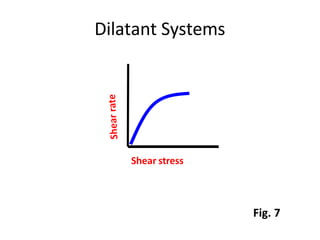
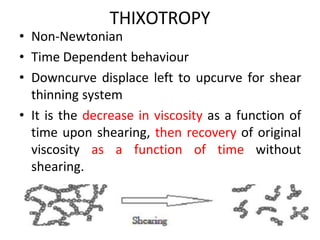
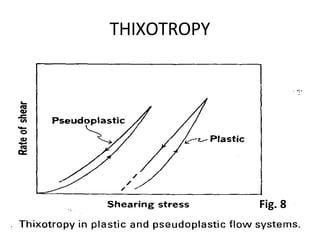
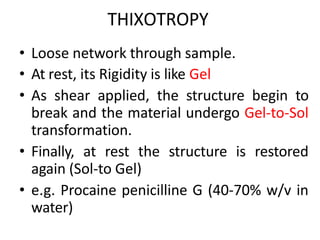
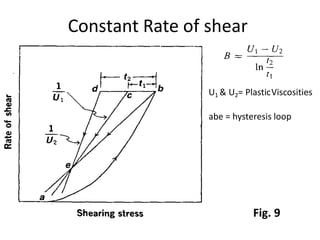
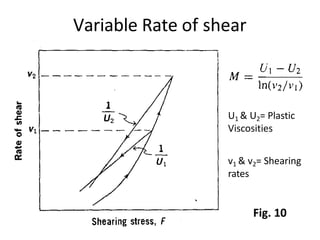
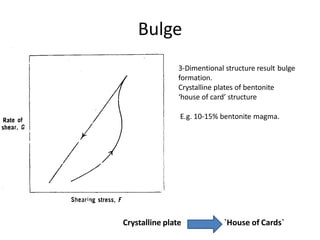
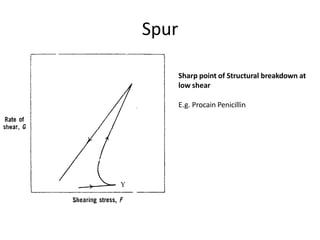
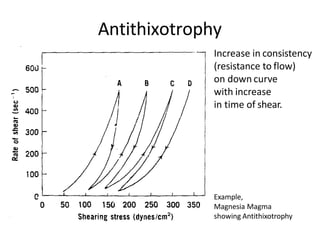
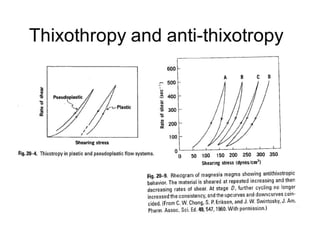
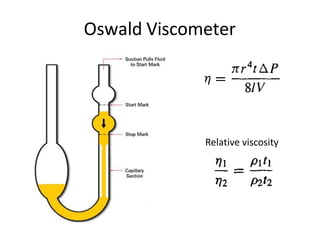
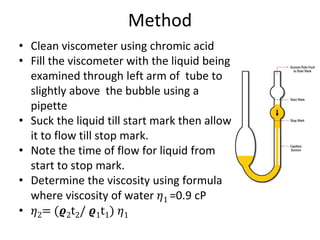
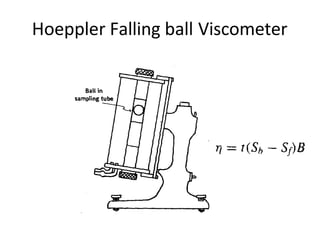
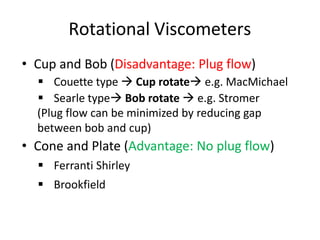
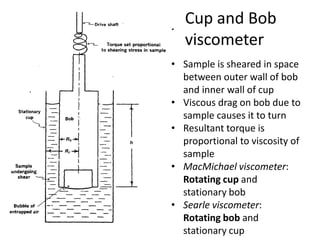
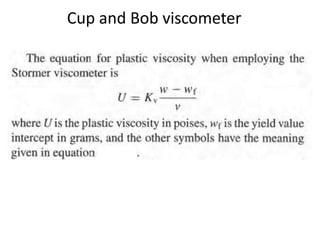
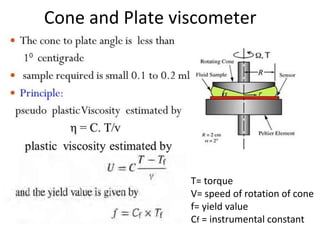
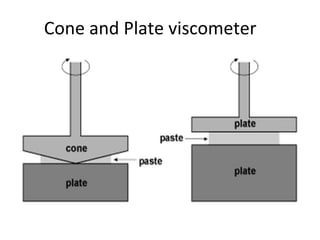
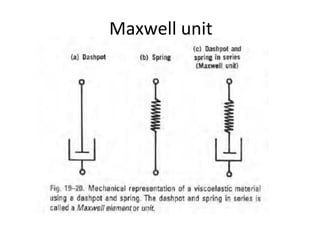
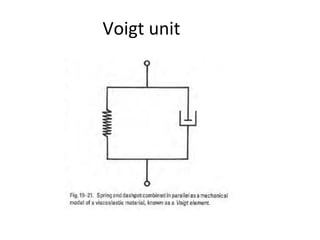
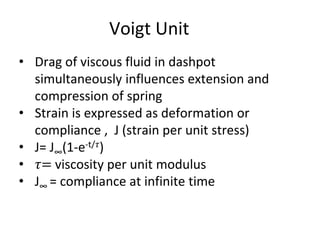
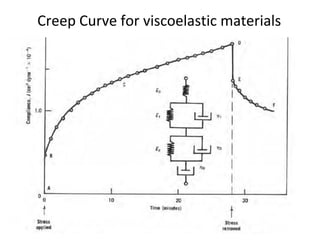
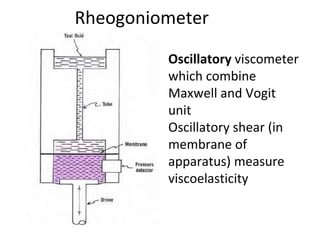
Rheology is the science describing the flow and deformation of matter under stress. Viscosity describes a fluid's resistance to flow and is dependent on factors like temperature, pressure, and shear rate. Newtonian fluids have a constant viscosity regardless of shear rate, while non-Newtonian fluids have variable viscosity. Common non-Newtonian behaviors include plastic flow, pseudoplastic (shear-thinning) flow, and dilatant (shear-thickening) flow. Thixotropy is time-dependent shear thinning where viscosity decreases under shear and recovers with time. Viscometers like capillary, falling ball, and rotational viscometers are used to measure viscosity. Rheology is important in formulation development