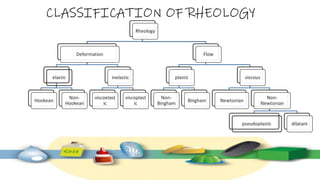
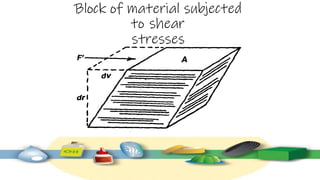
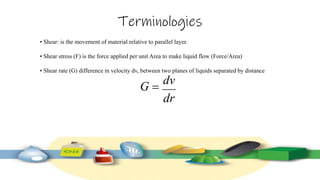
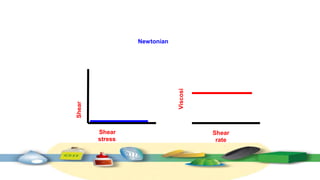
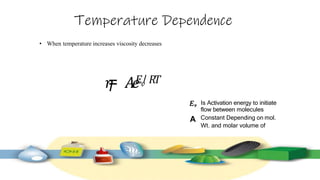
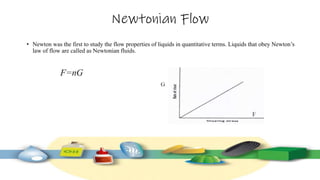
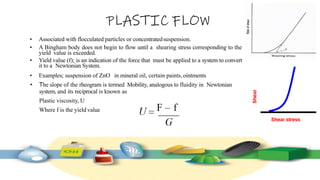
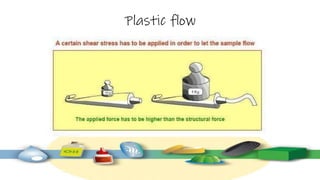
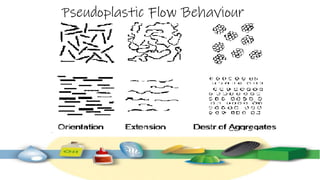
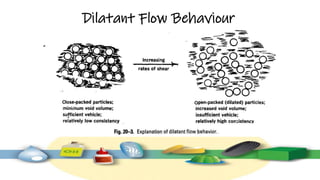
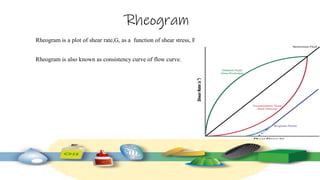
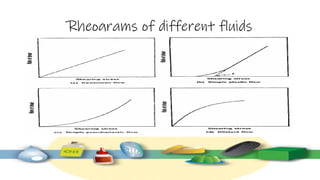
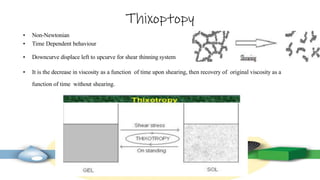
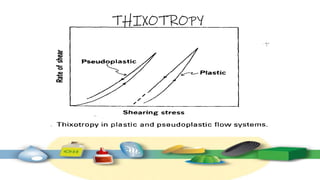
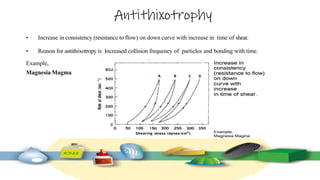
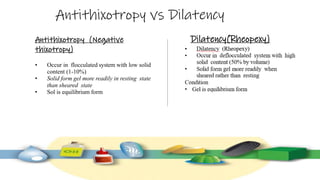
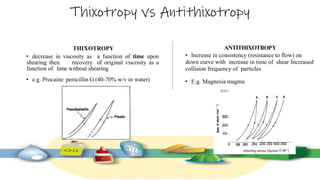
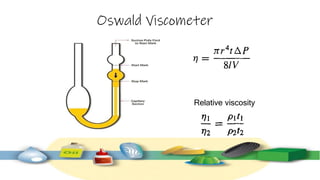
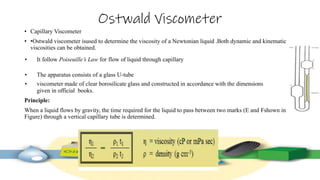
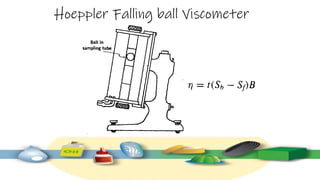
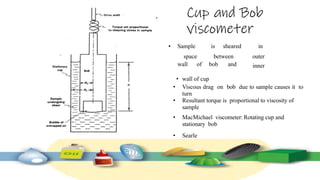
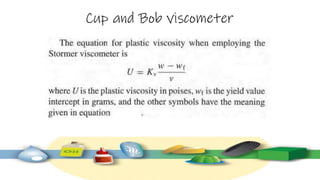
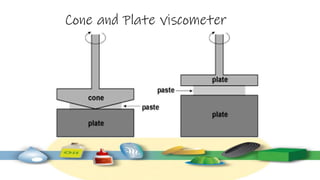
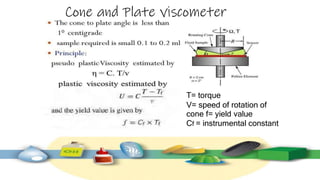
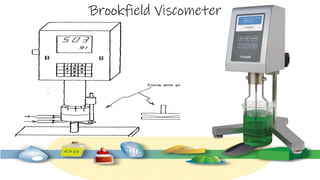
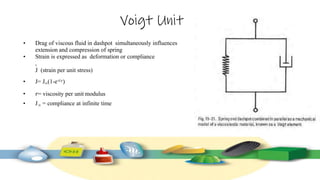
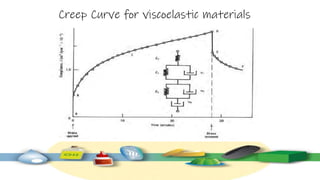
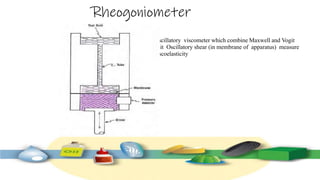
Rheology is the study of the flow and deformation of materials under stress, applicable to all substances from gases to solids. It covers Newtonian and non-Newtonian fluids, viscosity measurements, and applications in pharmaceuticals, including the formulation of various dosage forms. Key concepts include the effects of temperature on viscosity, different types of flow (e.g., plastic, pseudoplastic, and dilatant), and methods to measure viscosity using devices like capillary and rotational viscometers.