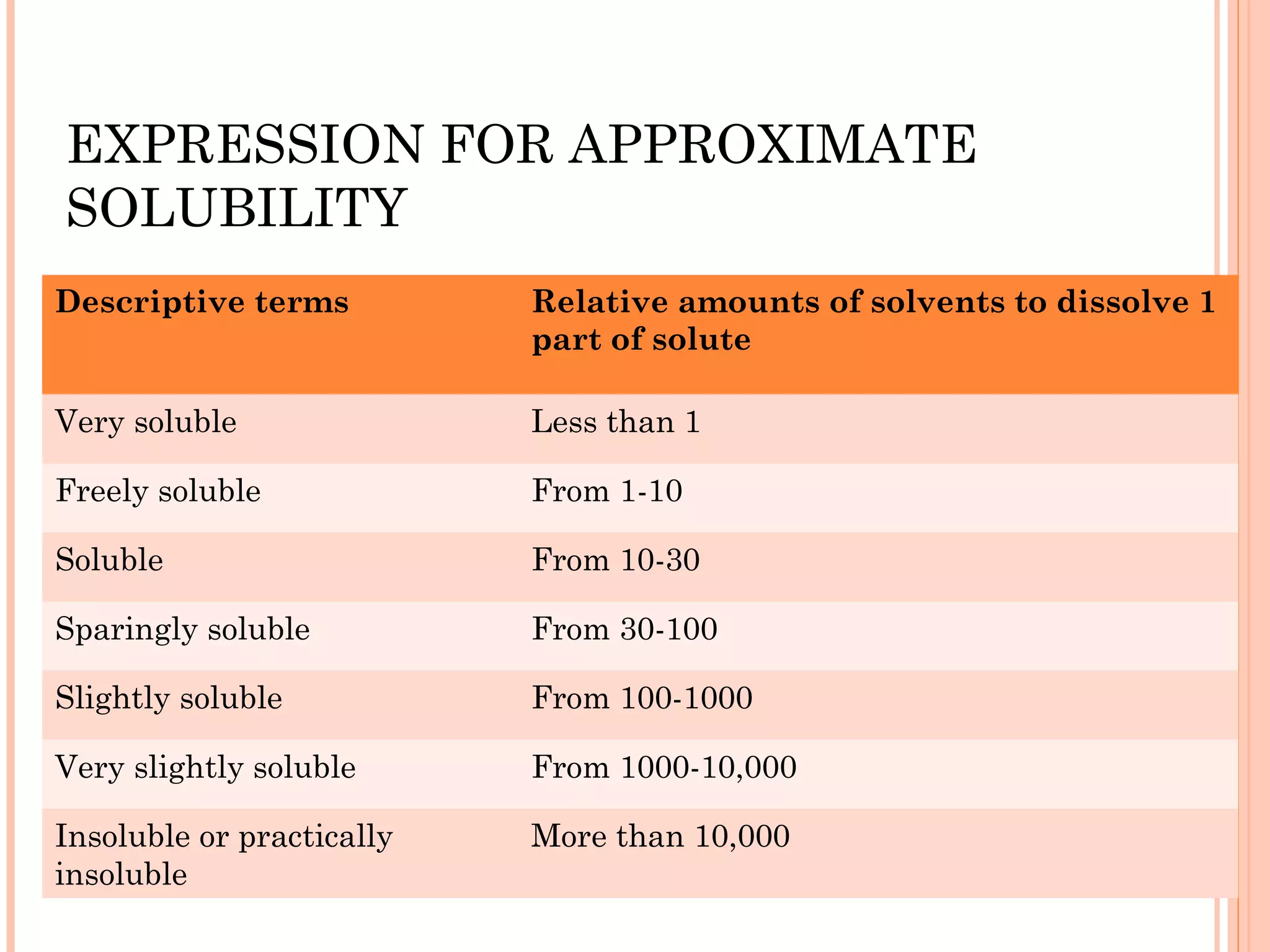
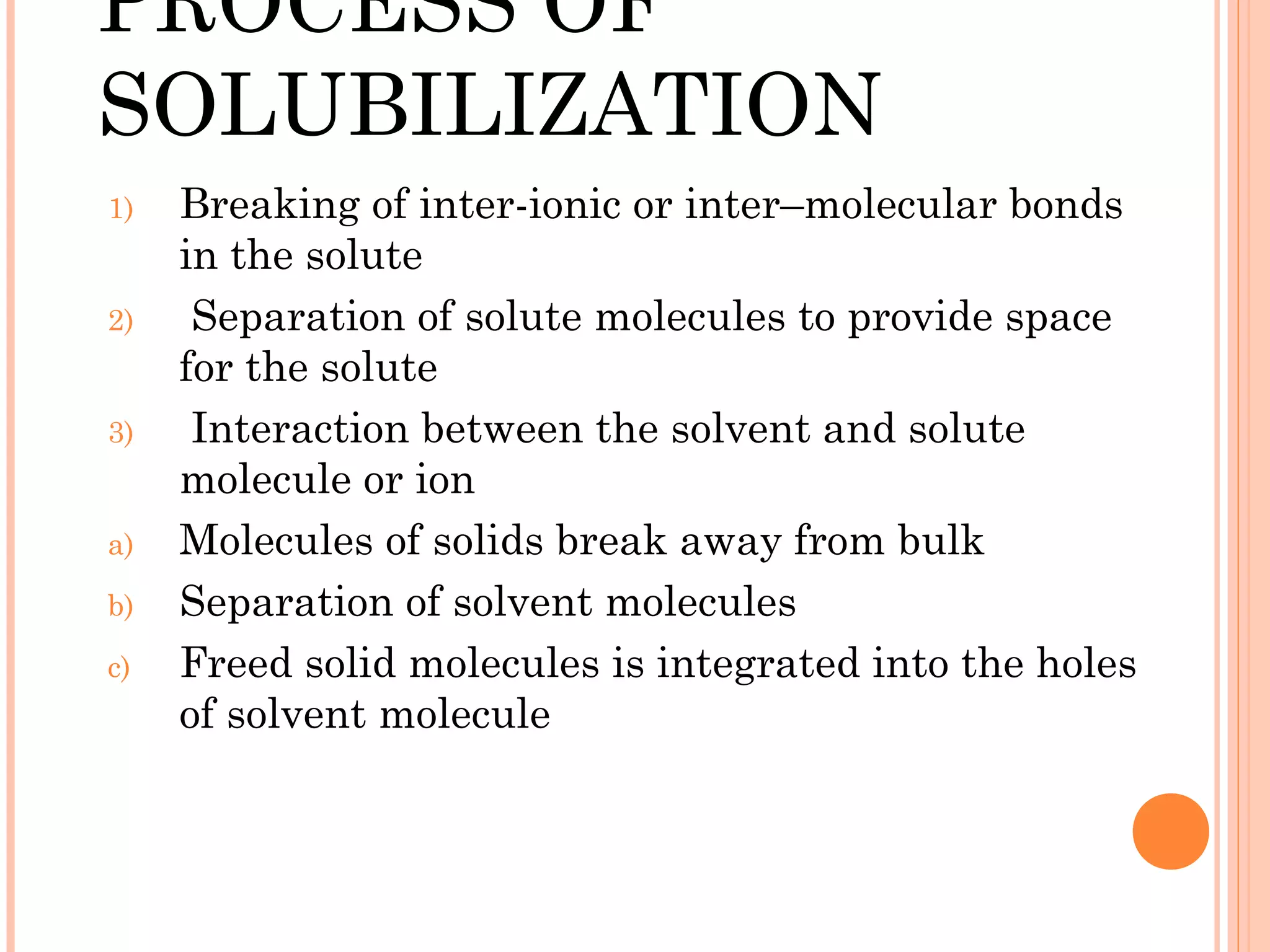
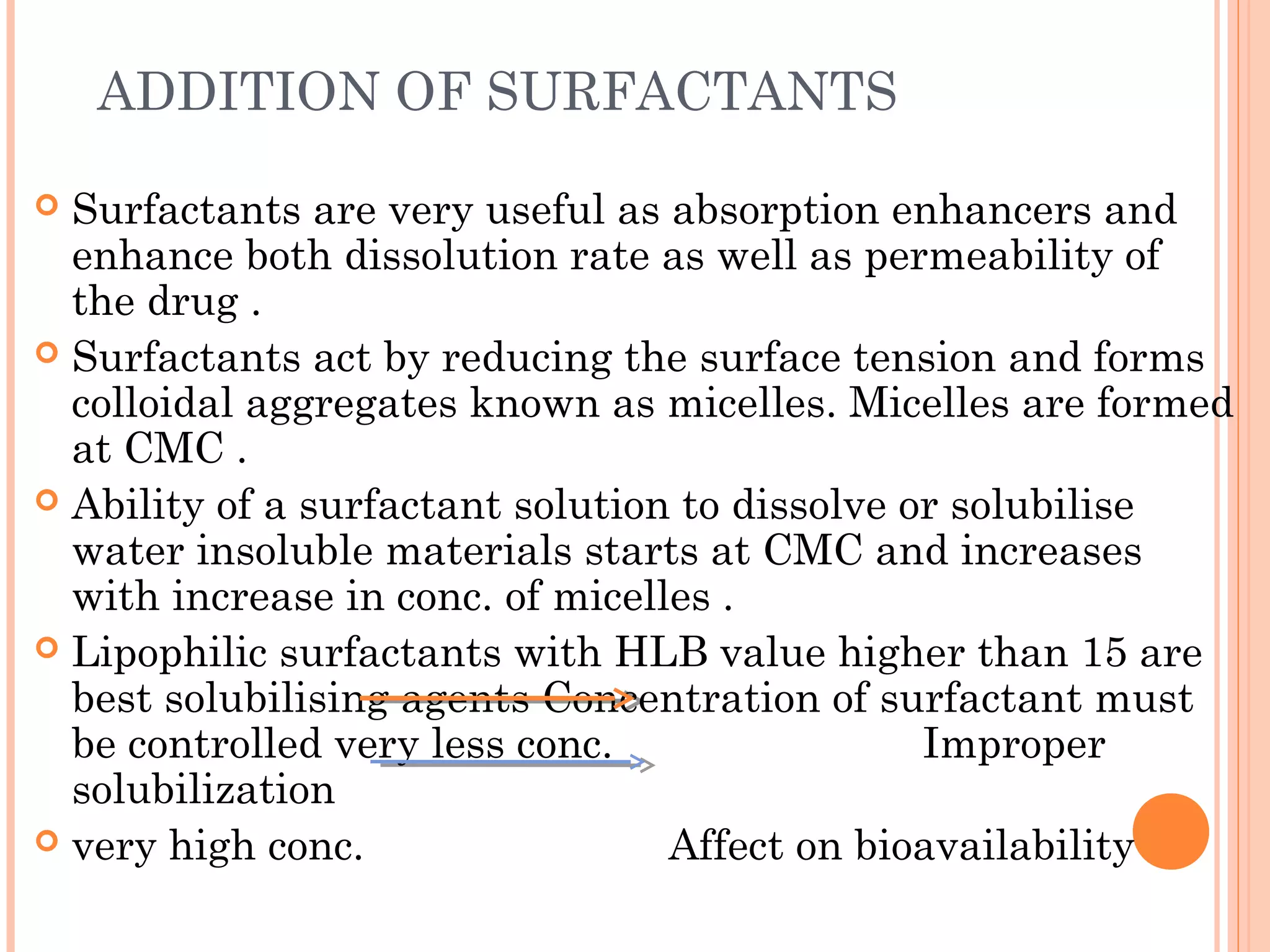
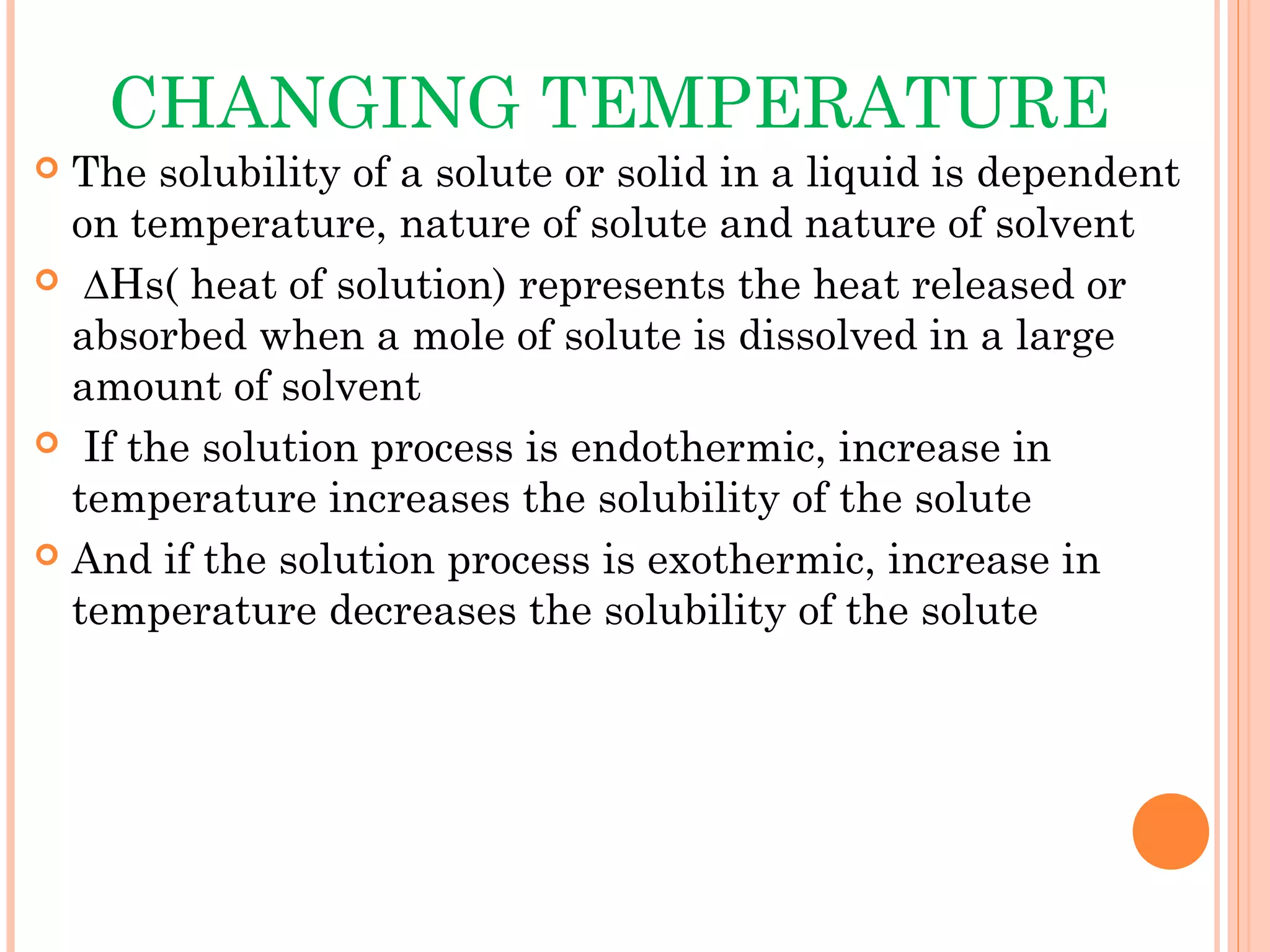
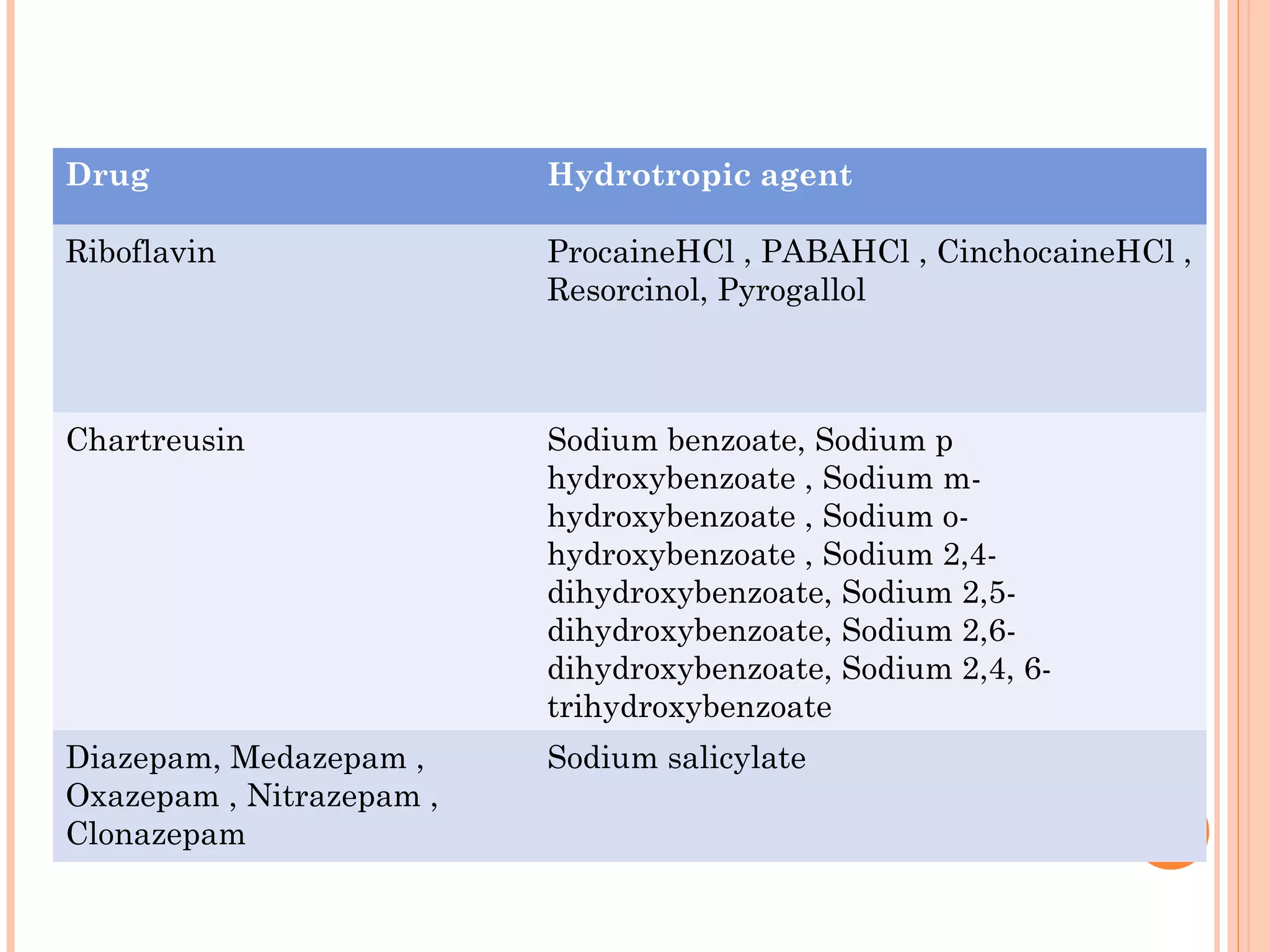
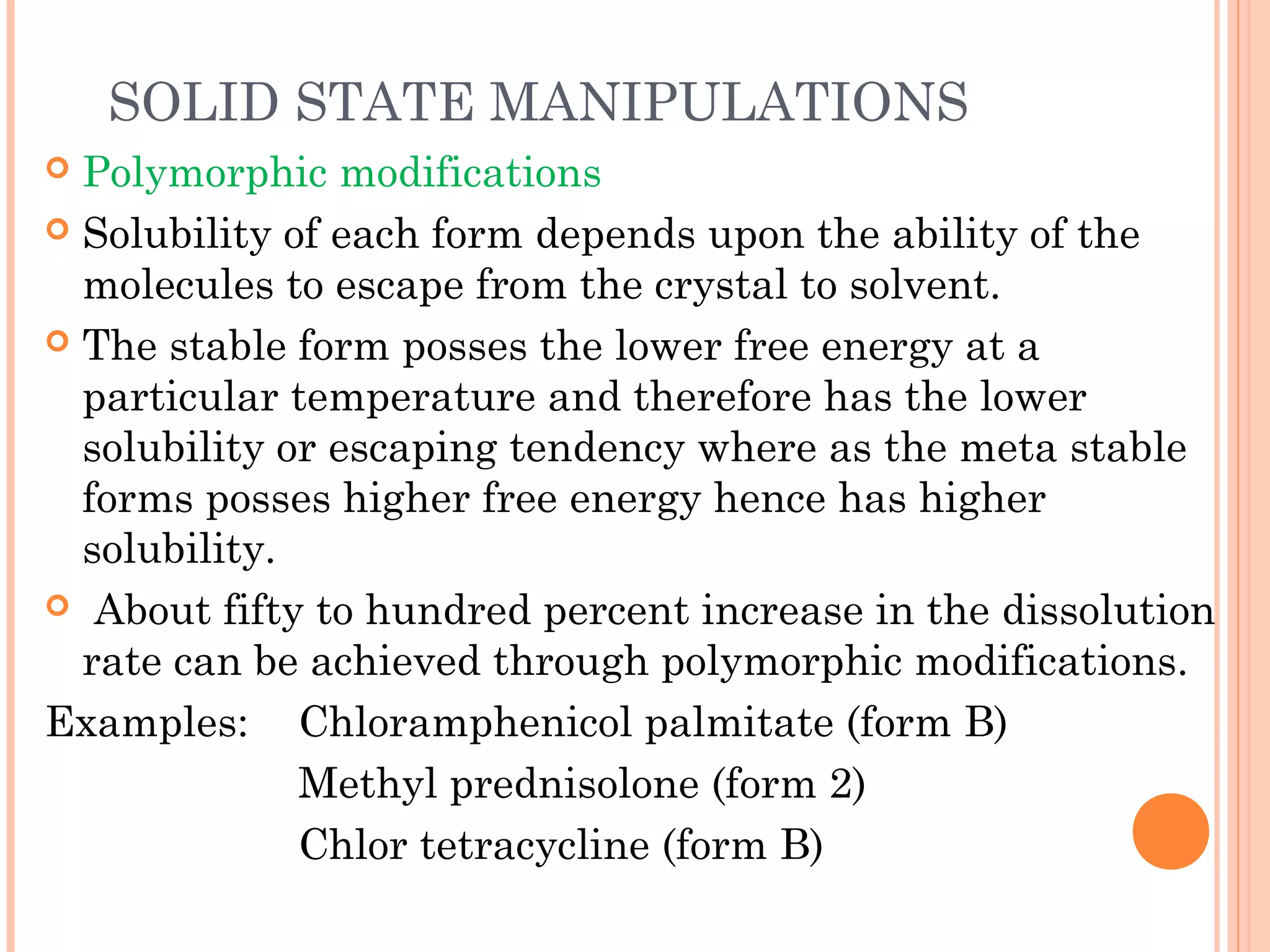
This document discusses various techniques to improve the solubility of poorly soluble drugs, which is important for developing effective dosage forms and achieving desired drug concentrations. It defines solubility and discusses the importance of solubility in drug development. Some key techniques covered are co-solvency, use of surfactants, solid dispersions, complexation, changing temperature, hydrotropy, polymorphism, amorphous forms, solvates, salt formation, and micronization/nanonization. The goal is to select the optimal method for a given drug to enhance dissolution and absorption.