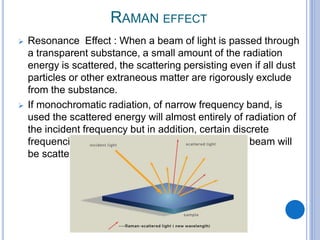
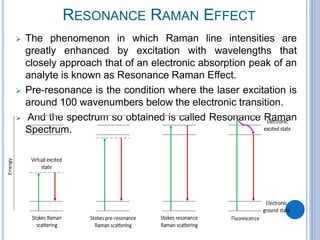
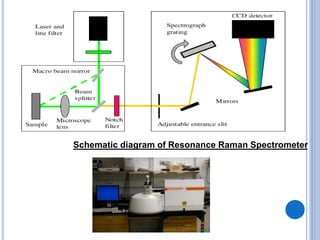
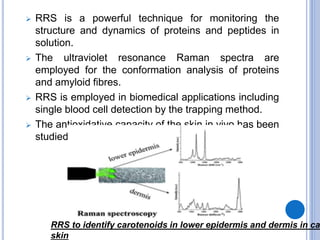
This document provides an overview of resonance Raman spectroscopy. It begins with introductions to Raman spectroscopy and the resonance Raman effect. It then covers the theory, instrumentation, applications to analyzing pentacene, and differences in spectra with different excitation wavelengths. Key advantages of resonance Raman spectroscopy include improved sensitivity and no need for additional probes. It concludes that tunable lasers allow analysis of multiple samples with different resonance wavelengths.