Embed presentation
Downloaded 14 times


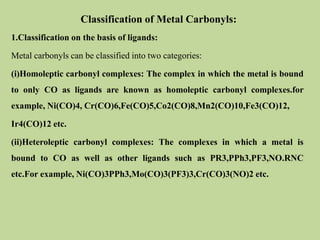




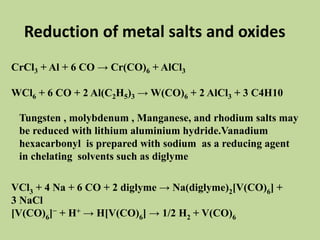
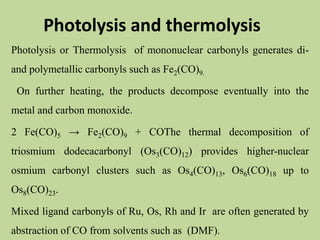
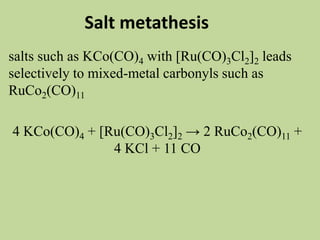

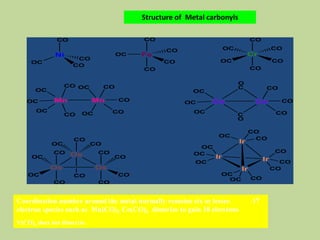
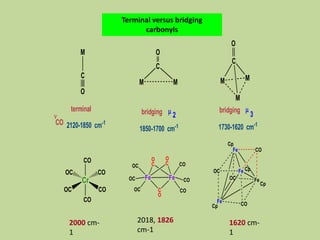
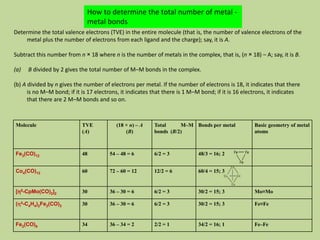




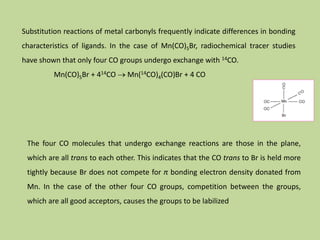

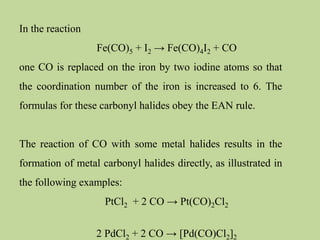

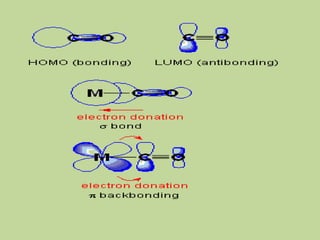
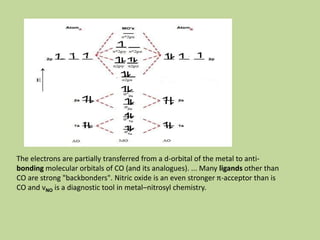






Transition metal carbonyls form when carbon monoxide bonds to a transition metal through both sigma and pi bonding. This synergistic metal-ligand bonding strengthens the metal-carbon bond. Metal carbonyls can be classified based on the ligands present and the number/structure of metal atoms. They exhibit a variety of reactions including substitution, reactions with halogens, and disproportionation. Metal carbonyls display properties related to their toxicity, magnetic behavior, thermal stability, and thermodynamic instability.