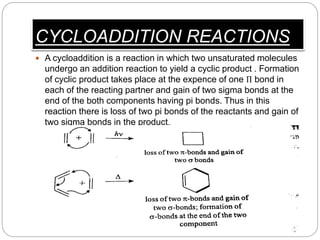
This document summarizes different types of cycloaddition reactions. It begins by defining cycloaddition reactions as those where two unsaturated molecules react to form a cyclic product through the loss of two pi bonds and gain of two sigma bonds. It then classifies cycloaddition reactions based on the number of pi electrons involved - [2+2], [4+2], and 1,3-dipolar cycloadditions. Examples of each type are provided along with diagrams to illustrate the reaction mechanisms and orbital interactions. Applications of cycloadditions in organic synthesis are also briefly mentioned.

![CONTENTS
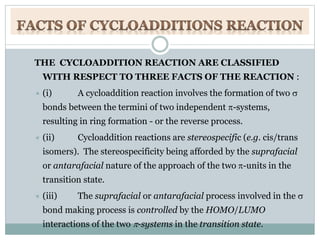
• Cycloaddition reaction.
• Facts of cycloaddition reaction.
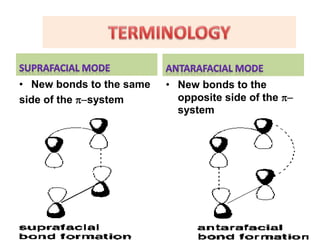
• Terminology- supra-antra mode
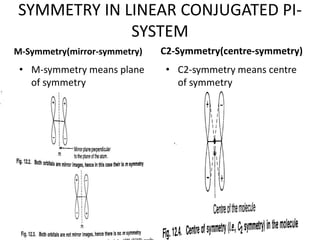
Symmetry
• F.M.O method.
• [ 2+2 ] cycloaddition reaction.
• [4+2 ] cycloaddition reaction.
• 1,3 dipolar cycloaddition reaction.
• Application of cycloadditions.
• Reference](https://image.slidesharecdn.com/qv4m1q9wslynamjr9lbp-cycloaddition-maisha-kanwar-sem-2-230203153752-12365b24/85/Cycloaddition-Reactions-2-320.jpg)
![FMO METHOD
In order for a cycloaddition to occur,must be bonding overlap
between p orbital at the terminal carbons of each electron system
,this is where the new sigma-bonds are formed. Lets us explain this
point with a [4+2] cycloaddition. Let us suppose that diene (4-
component) behaves as electron donar and the dienophile (2-
component)as the electron acceptor.](https://image.slidesharecdn.com/qv4m1q9wslynamjr9lbp-cycloaddition-maisha-kanwar-sem-2-230203153752-12365b24/85/Cycloaddition-Reactions-7-320.jpg)
![[2+2] CYCLOADDITION
REACTION
When 2 electron and 2 electron system
combine together to give cycloalkene the reaction
is called 2+2 cycloaddition it can occur suprafical
antrafacial mode of addition.](https://image.slidesharecdn.com/qv4m1q9wslynamjr9lbp-cycloaddition-maisha-kanwar-sem-2-230203153752-12365b24/85/Cycloaddition-Reactions-8-320.jpg)
![[2+2] CYCLOADDITIONS
THERMAL INDUCED](https://image.slidesharecdn.com/qv4m1q9wslynamjr9lbp-cycloaddition-maisha-kanwar-sem-2-230203153752-12365b24/85/Cycloaddition-Reactions-9-320.jpg)
![[2+2] CYCLOADDITIONS
• PHOTO INDUCED-](https://image.slidesharecdn.com/qv4m1q9wslynamjr9lbp-cycloaddition-maisha-kanwar-sem-2-230203153752-12365b24/85/Cycloaddition-Reactions-10-320.jpg)
![[4+2] CYCLOADDITION REACTION
• A cycloaddition reaction is the concerted bonding together of
two independent pi-electron systems to form a new ring of
atoms. ... The Diels-Alder cycloaddition is classified as a [4+2]
process because the diene has four pi-electrons that shift
position in the reaction and the dienophile has two.](https://image.slidesharecdn.com/qv4m1q9wslynamjr9lbp-cycloaddition-maisha-kanwar-sem-2-230203153752-12365b24/85/Cycloaddition-Reactions-11-320.jpg)
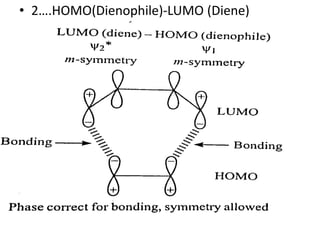
![[4+2] cycloaddition reaction
• THERMAL INDUCED REACTION –There are two
possible interactions, HOMO (diene) – LUMO
(dienophile) and HOMO(Dienophile)-LUMO(diene).
• 1.HOMO(Diene)-LUMO(dienophile)](https://image.slidesharecdn.com/qv4m1q9wslynamjr9lbp-cycloaddition-maisha-kanwar-sem-2-230203153752-12365b24/85/Cycloaddition-Reactions-12-320.jpg)
![[4+2] cycloaddition reaction
• PHOTO INDUCED REACTION-When diene is excited
by light.its HOMO becomes Ψ₃* which has m
symmetry and this MO cannot overlap with LUMO
of ethylene which has C₂ symmetry.
• Thus photochemical
Cycloaddition of [4+2]
Systemis symmetry
forbidden reaction.](https://image.slidesharecdn.com/qv4m1q9wslynamjr9lbp-cycloaddition-maisha-kanwar-sem-2-230203153752-12365b24/85/Cycloaddition-Reactions-14-320.jpg)
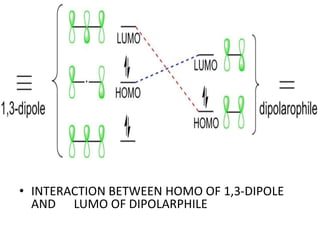
![1,3 –DIPOLAR CYCLOADDITIONS
• 1,3-Dipolar cycloadditions represent synthetically important
reactions for the preparation of heterocyclic five-membered
rings. They belong into the group of [4+2] cycloadditions
according to the Woodward-Hoffmann classification.
•](https://image.slidesharecdn.com/qv4m1q9wslynamjr9lbp-cycloaddition-maisha-kanwar-sem-2-230203153752-12365b24/85/Cycloaddition-Reactions-15-320.jpg)
![APPLICATIONS OF CYCLOADDITIONS
• N1 unit transfer reaction to C–C double bonds.
• [3+2] Cycloaddition of α, β-unsaturated metal-carbene
complexes.
• Formal [3+3] cycloaddition approach to natural product
synthesis.
• Development of new methods for the construction of
heterocycles based on cycloaddition reaction of 1,3-
dipoles.
• Cycloreversion approach for preparation of large π-
conjugated compounds.
• Transition metal-catalyzed or mediated [5+1]
cycloadditions.
https://www.wiley.com/en-
us/Methods+and+Applications+of+Cycloaddition+Reactions+in+Organic+Syntheses-p-9781118299883](https://image.slidesharecdn.com/qv4m1q9wslynamjr9lbp-cycloaddition-maisha-kanwar-sem-2-230203153752-12365b24/85/Cycloaddition-Reactions-17-320.jpg)