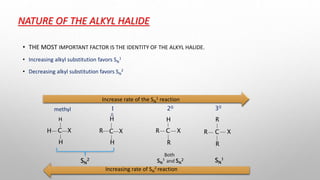
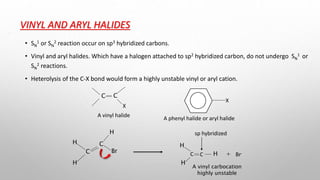
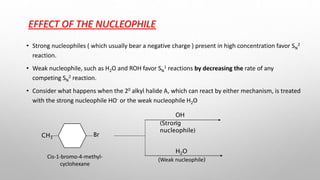
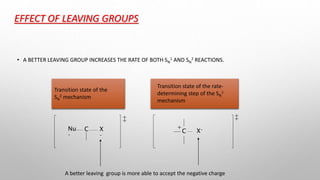
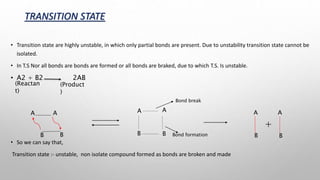
The document discusses Hammond's postulates, which state that the transition state of a chemical reaction resembles the structure of the species (reactant or product) that is closer in energy. Specifically:
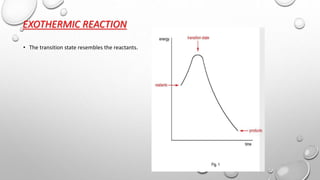
1) For exothermic reactions, the transition state resembles the reactants more than the products.
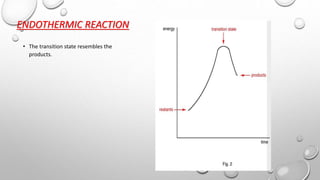
2) For endothermic reactions, the transition state resembles the products more than the reactants.
3) Hammond's postulates can be used to predict reaction mechanisms and explain factors that influence reaction rates.






![APPLICATION OF THE HAMMOND’S POSTULATE
TO THE SN
1 REACTION
• Since CH3
+ is less stable than (CH3)3C+
• En [1] > En [2]
• Reaction [1] is slower
Fig.
Energy diagram for carbocation
formation in two different SN
1
reactions](https://image.slidesharecdn.com/hammondspostulates-220126150459/85/Hammonds-postulates-10-320.jpg)