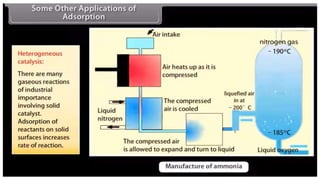
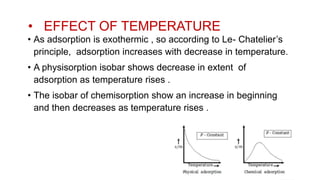
This document discusses adsorption, which is the process where molecules of gas or liquid adhere to a surface. It defines key terms like adsorbent, adsorbate, and sorption. It describes the mechanisms of physisorption and chemisorption. Finally, it outlines several factors that affect the extent of adsorption, such as the nature and surface area of the adsorbent, pressure and temperature of the system, and activation of the adsorbent.