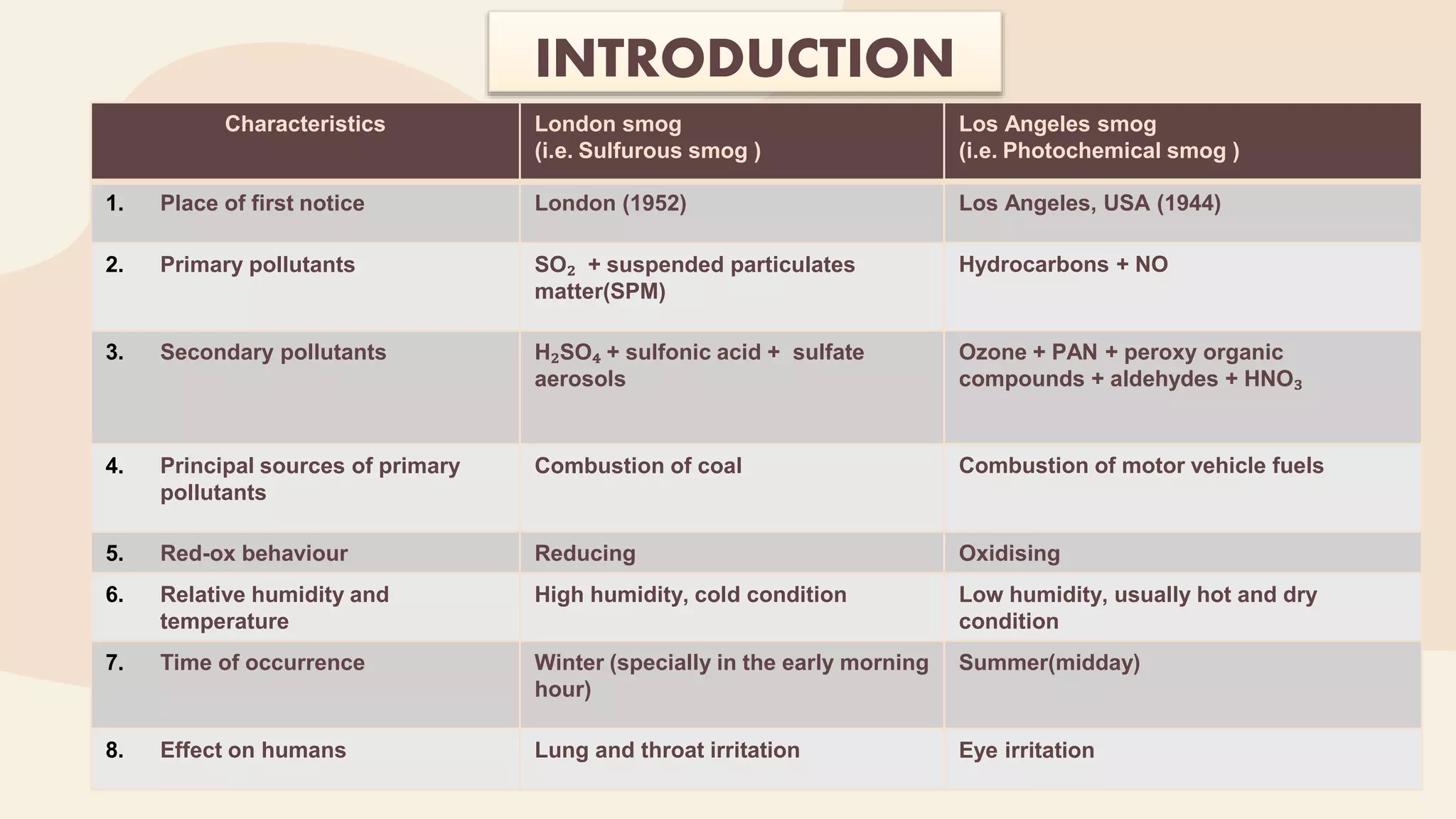
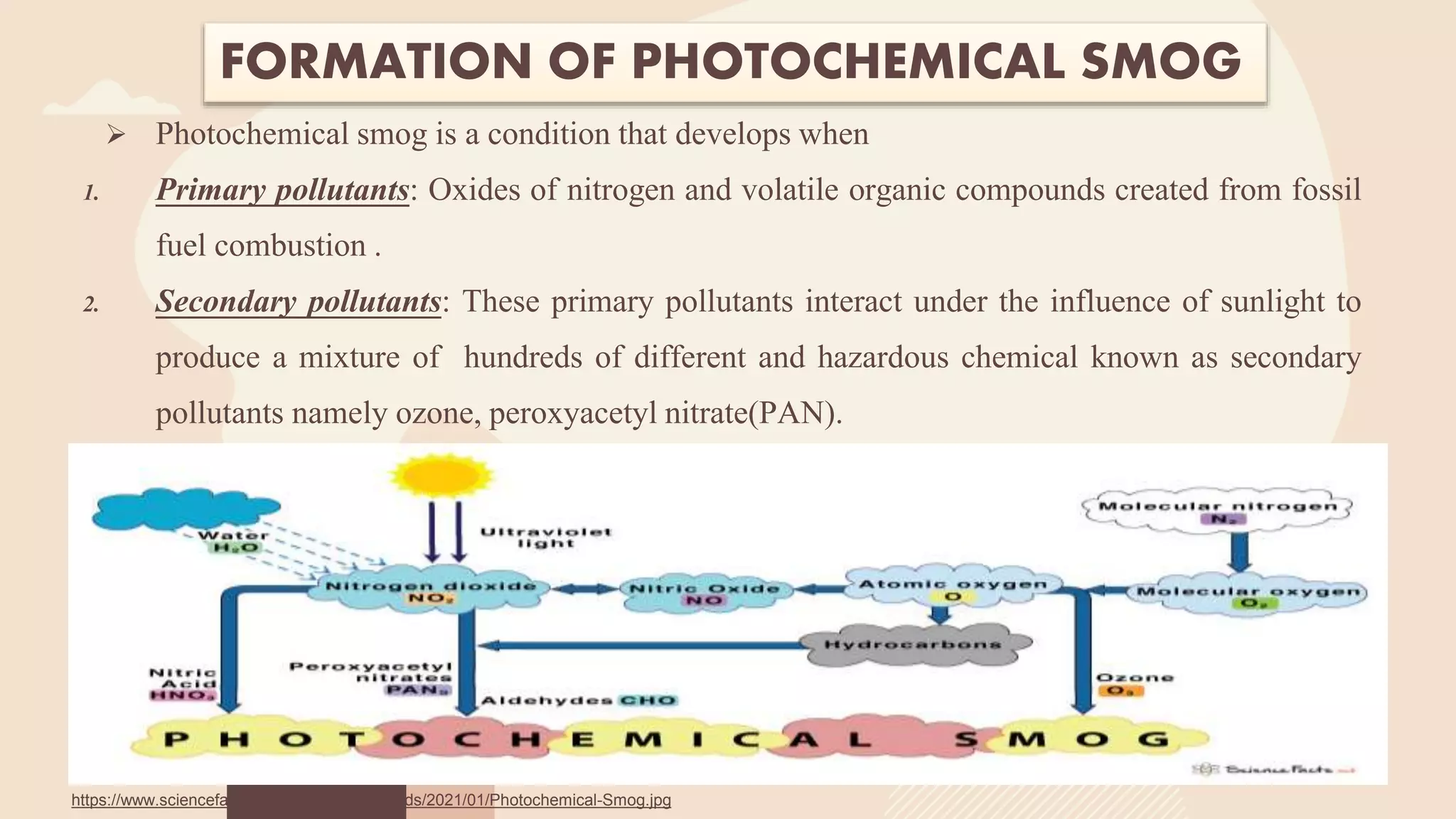
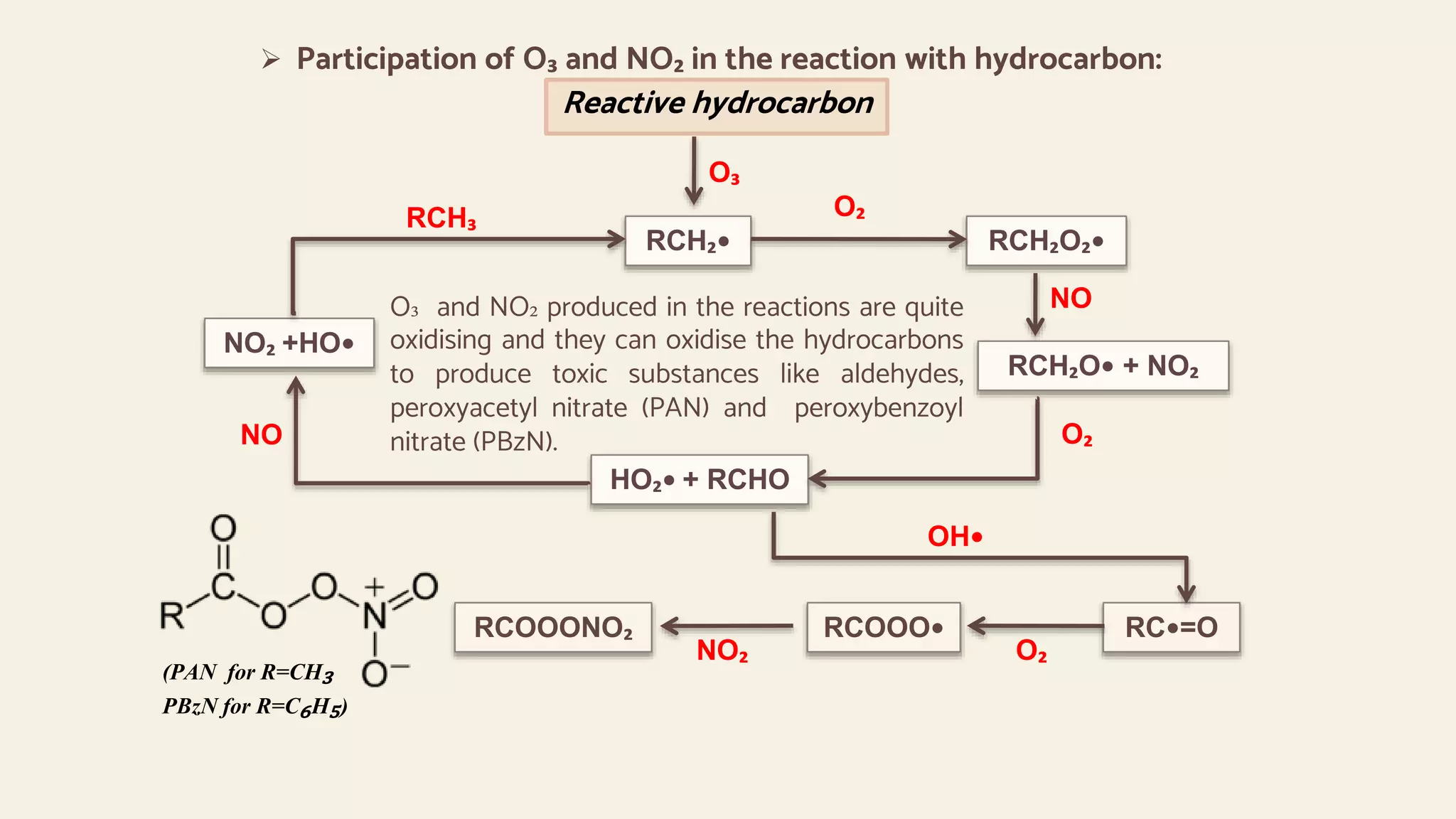
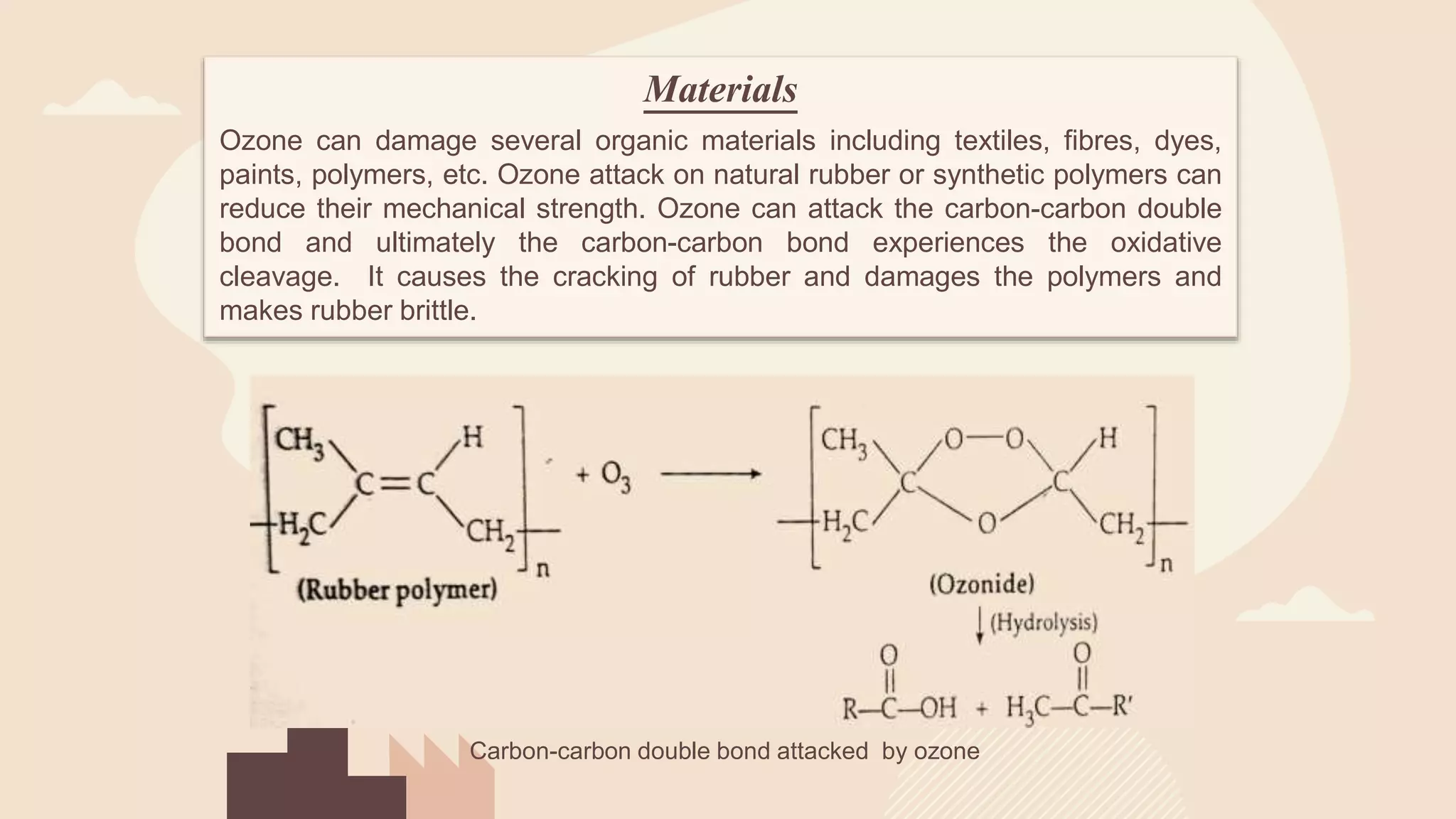
This document provides an overview of photochemical air pollution. It begins with historical context on London smog and Los Angeles smog. It then discusses the formation of photochemical smog through primary pollutants like NOx and VOCs interacting under sunlight to form secondary pollutants such as ozone. The major sources of these primary pollutants are also identified as fossil fuel combustion from automobiles and industry. Effects of photochemical air pollution on health, plants, and materials are outlined. Finally, control methods are summarized, including use of catalytic converters to reduce NOx and VOC emissions from vehicles.