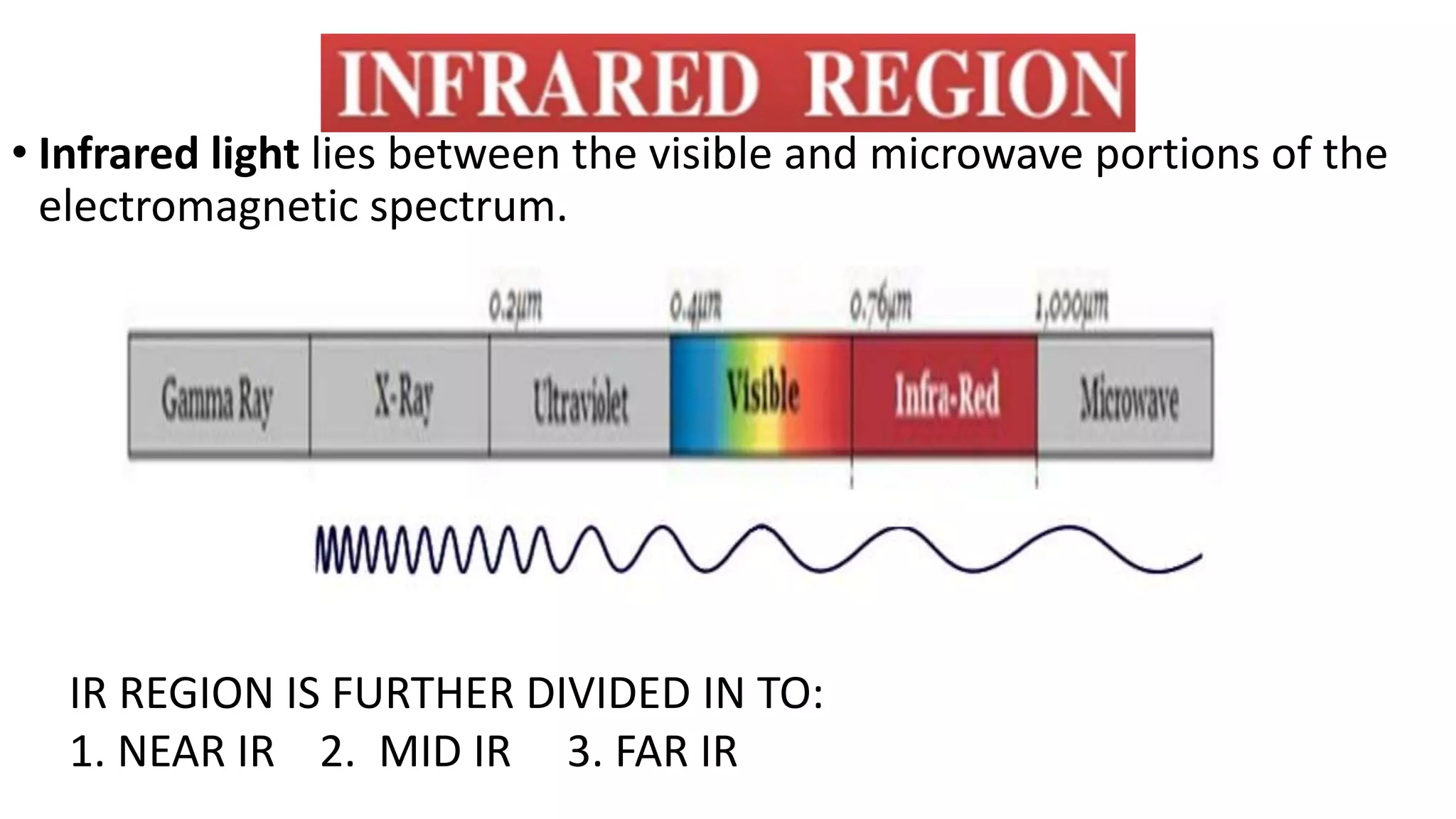
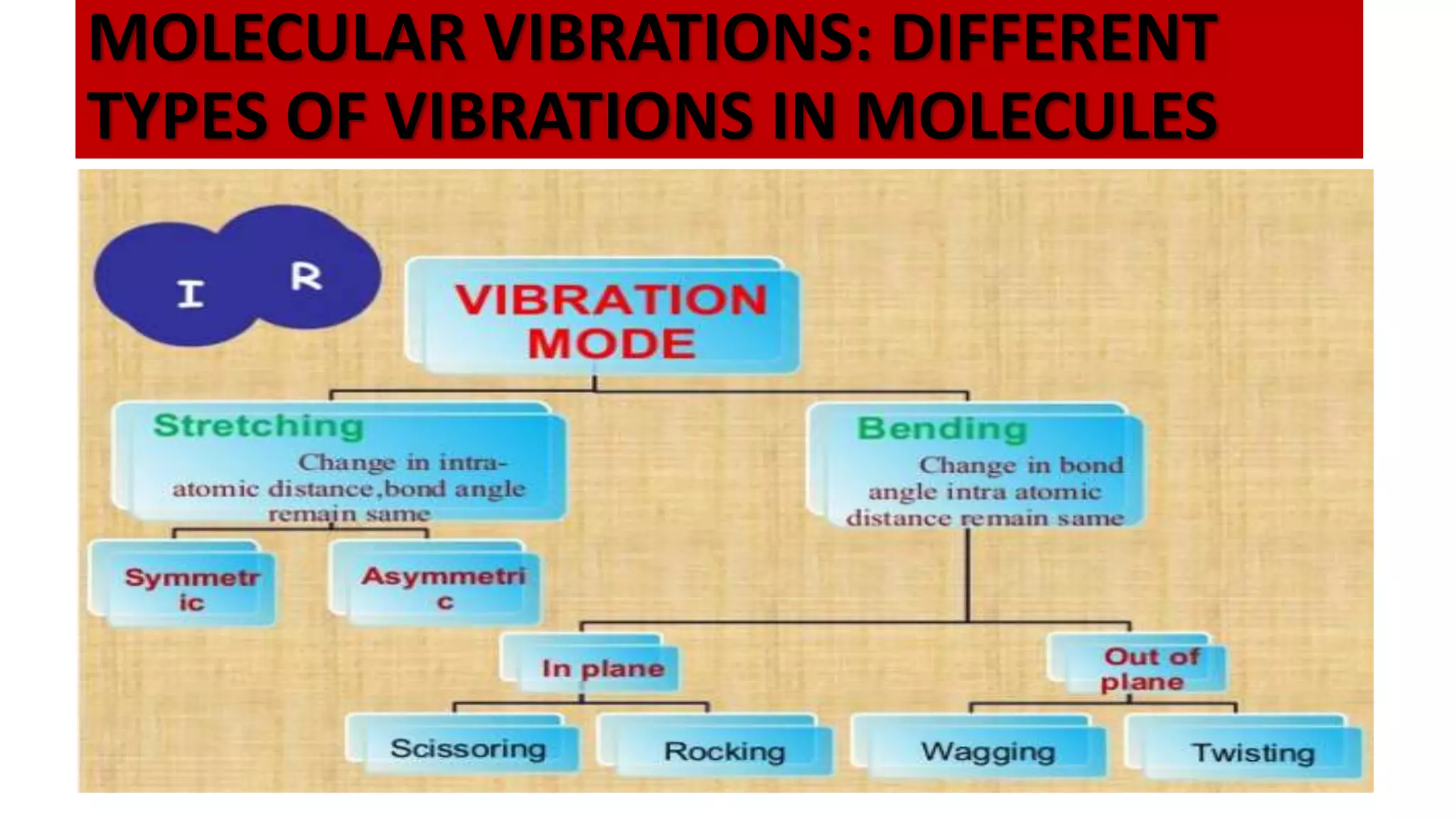
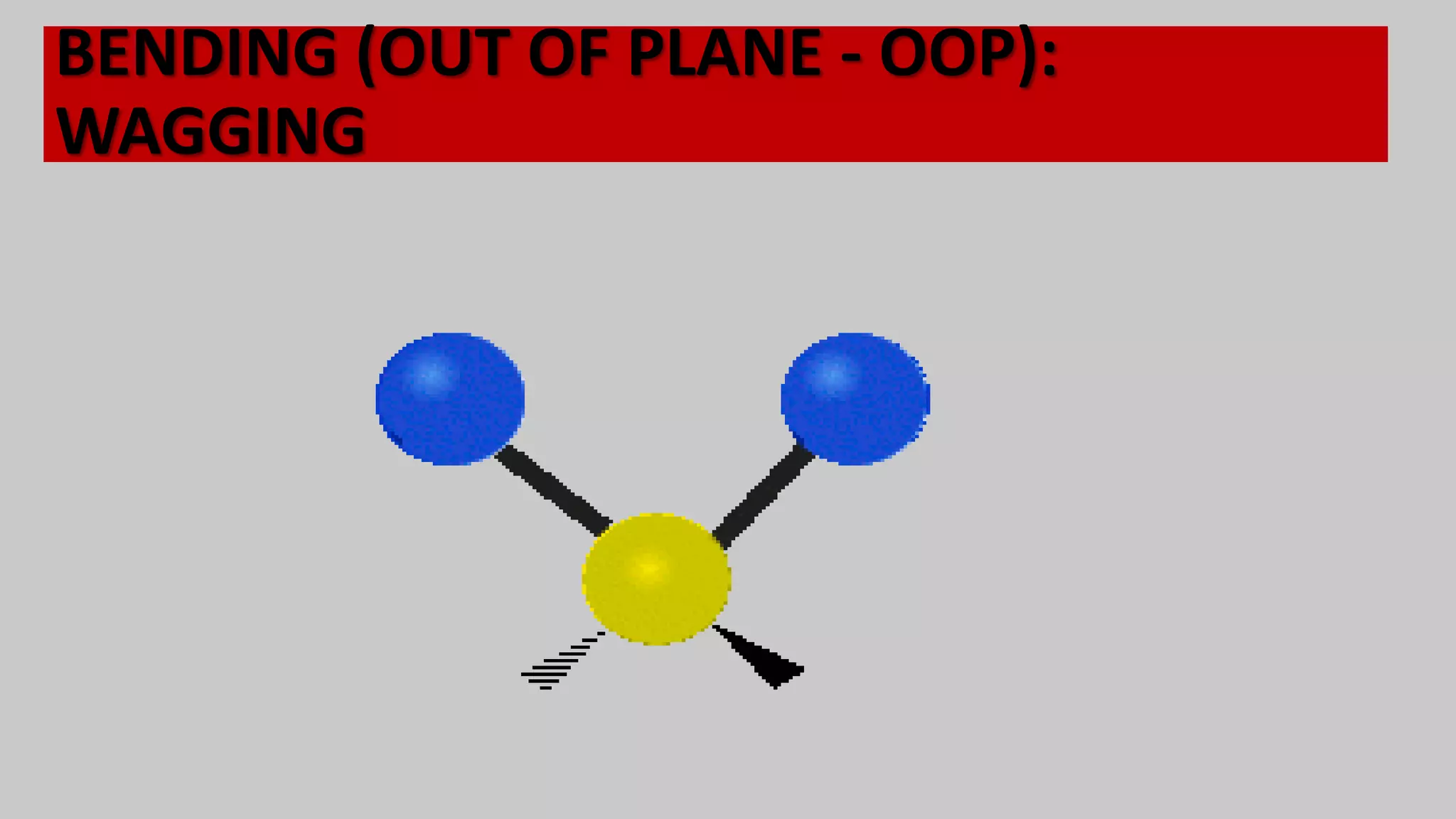
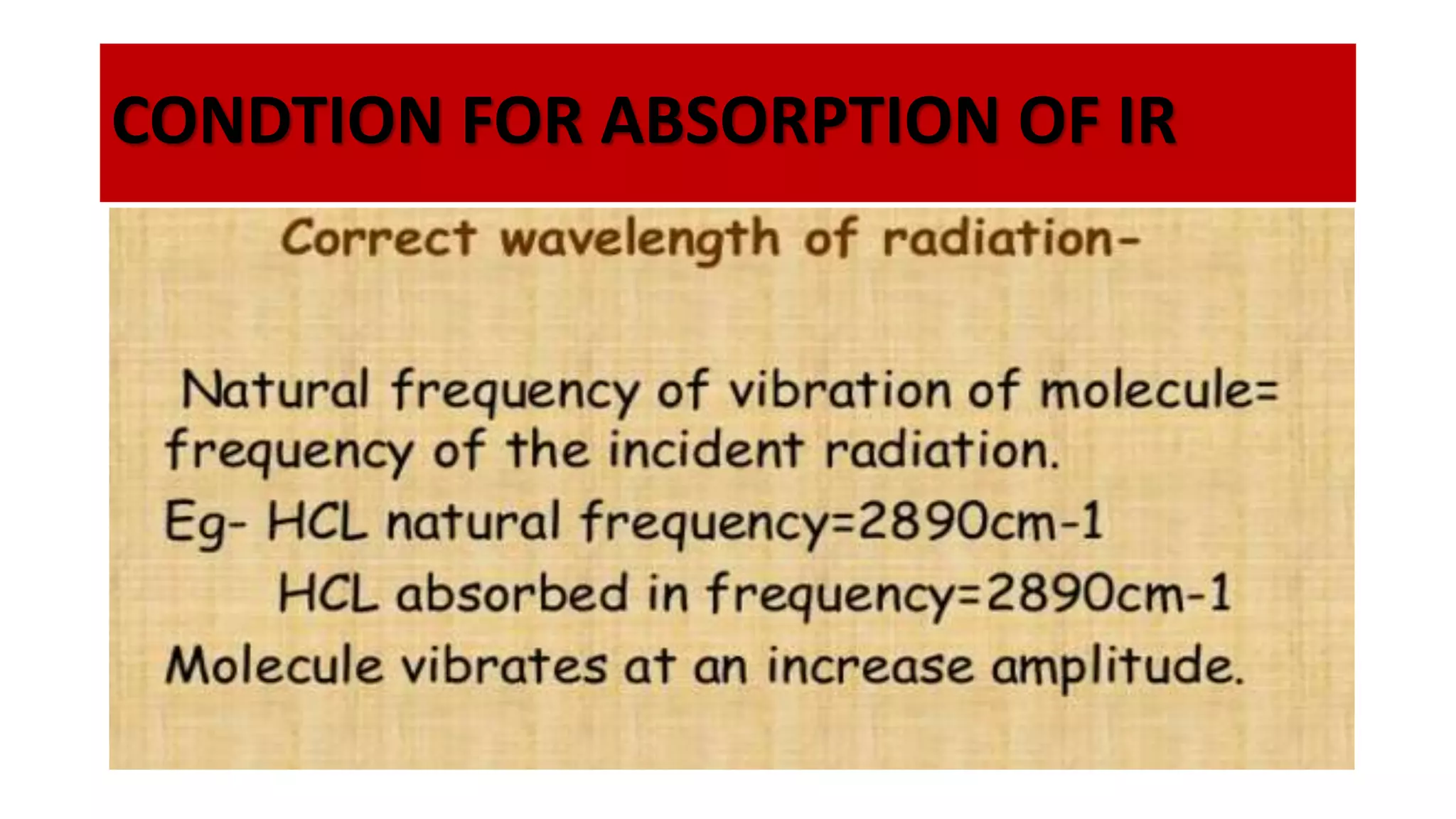
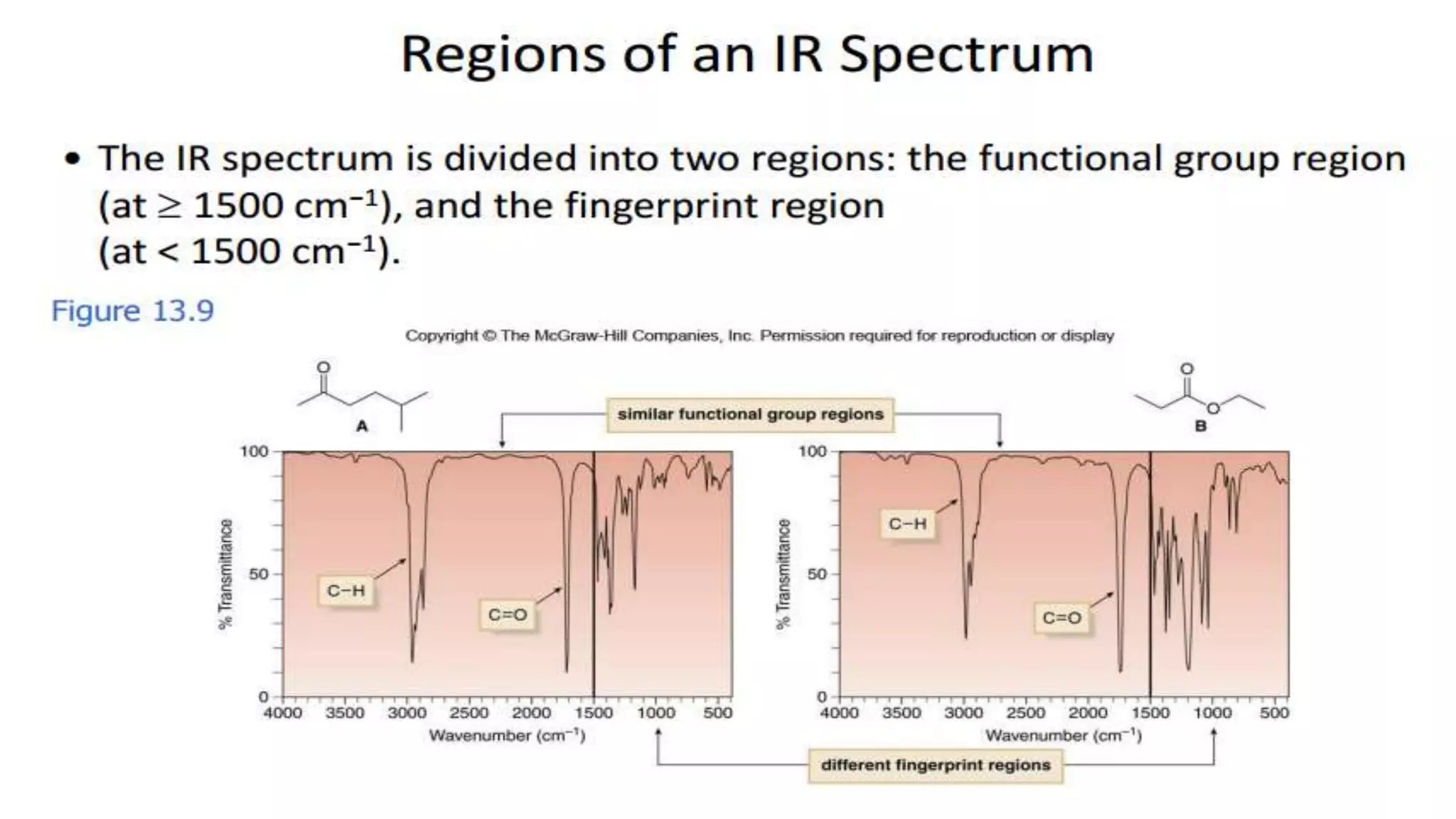
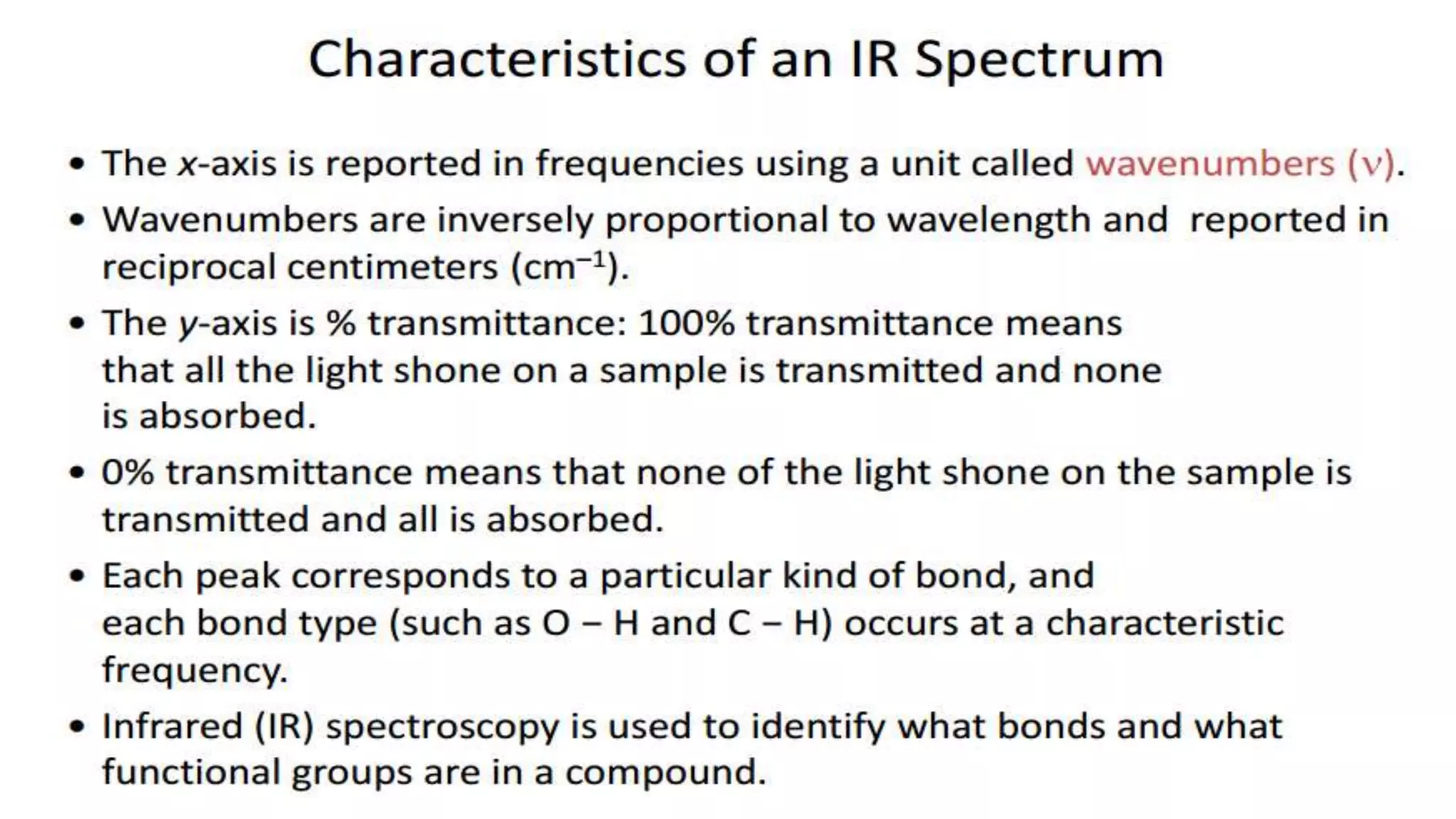
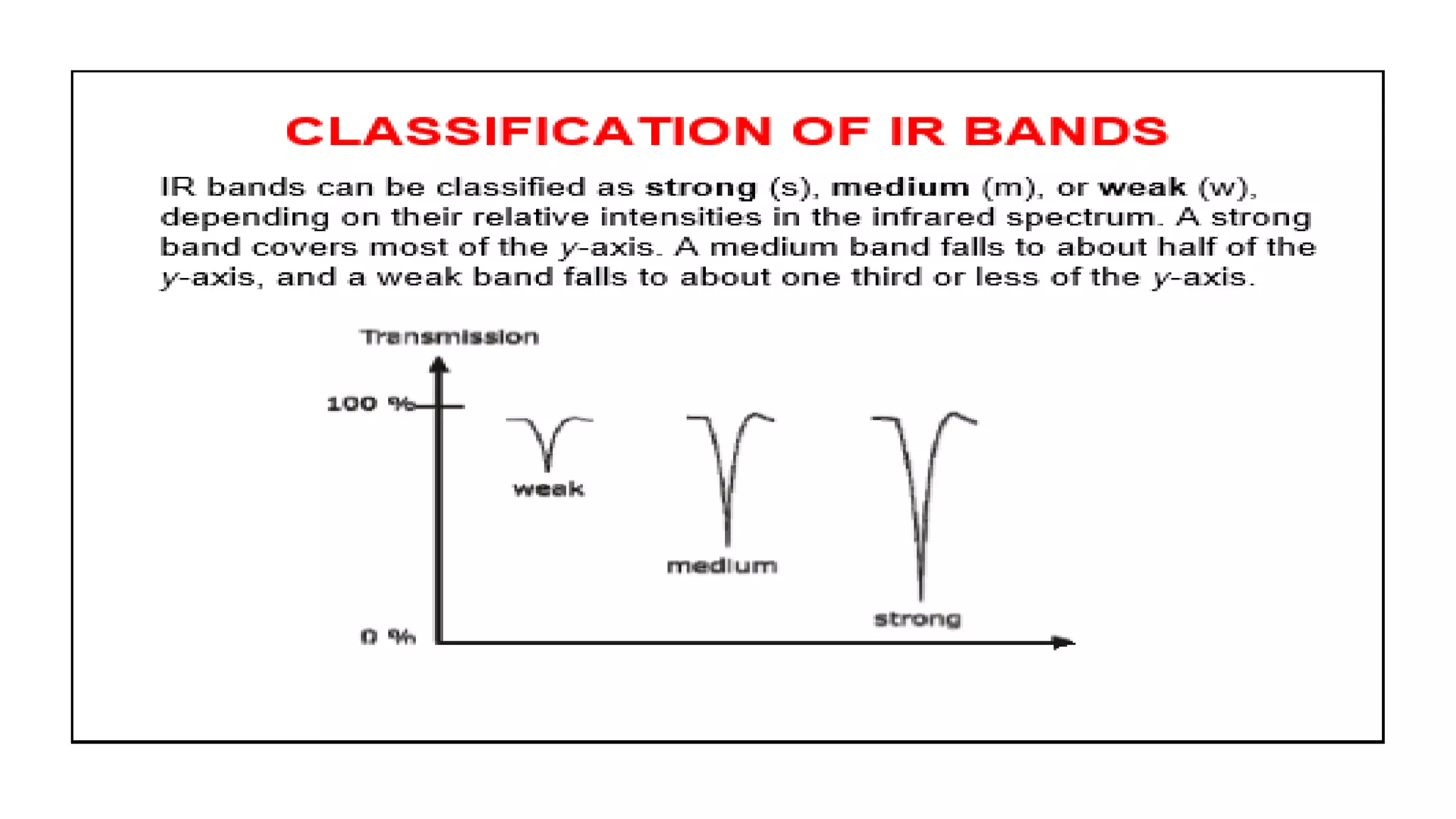
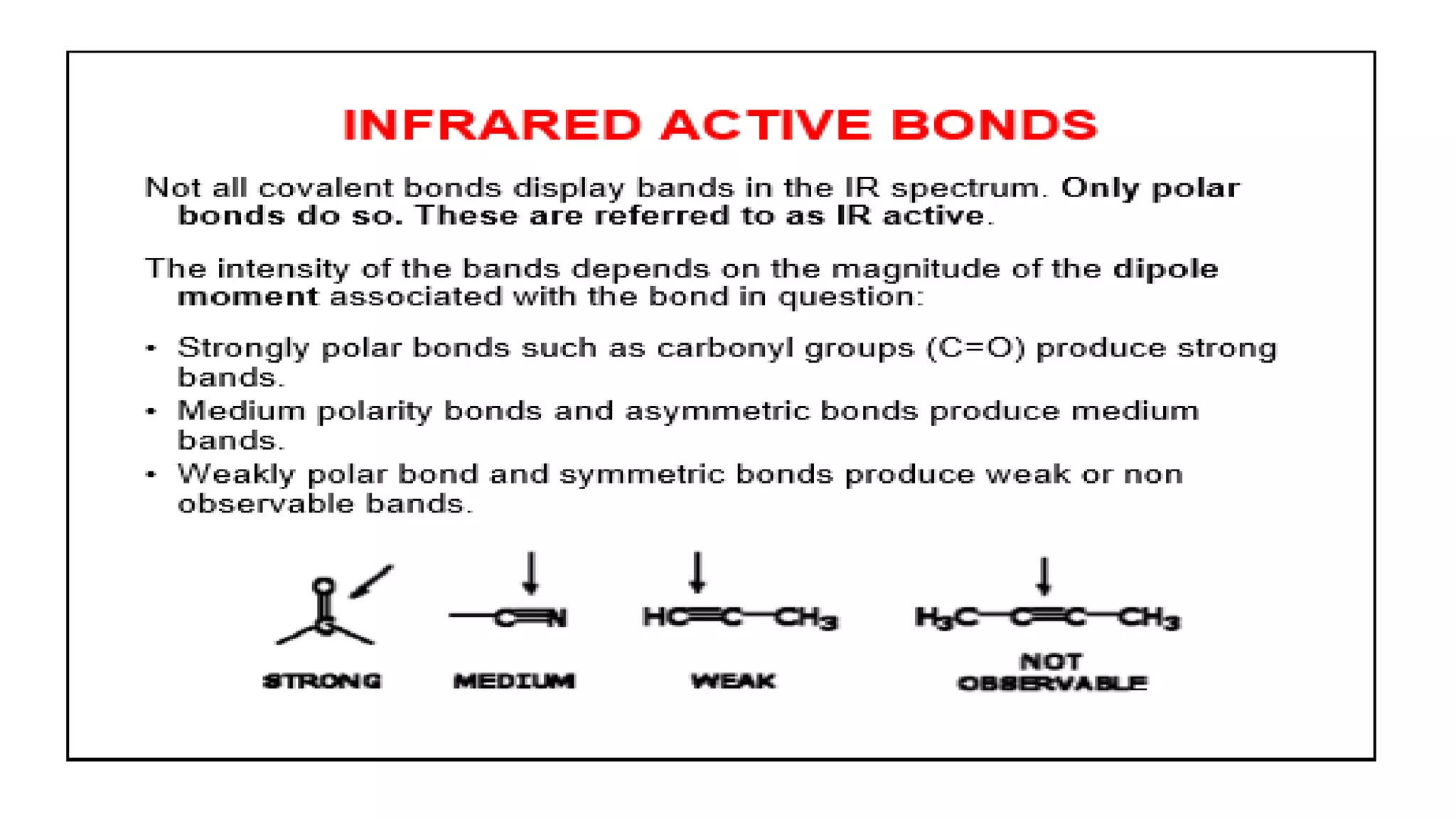
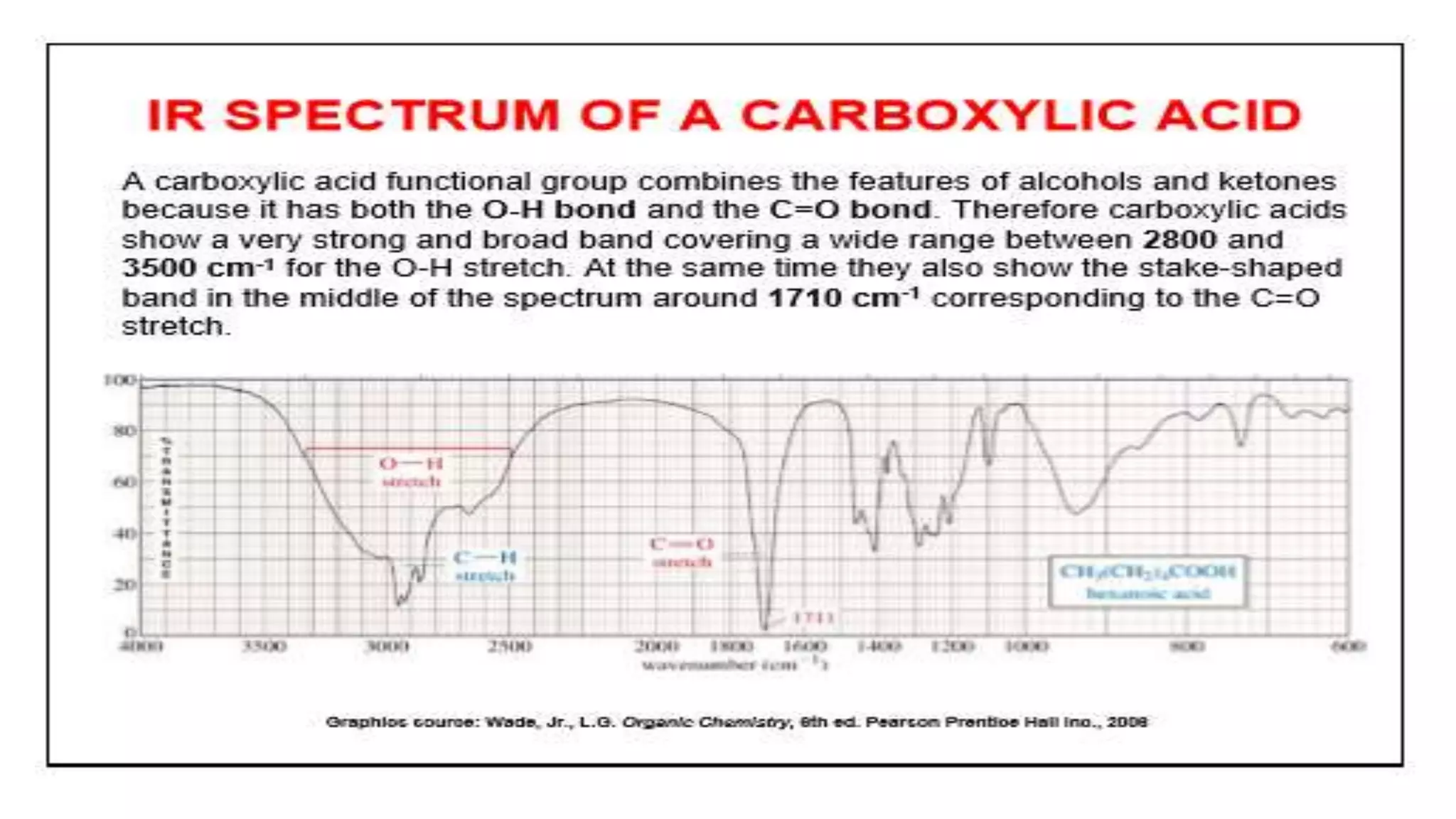
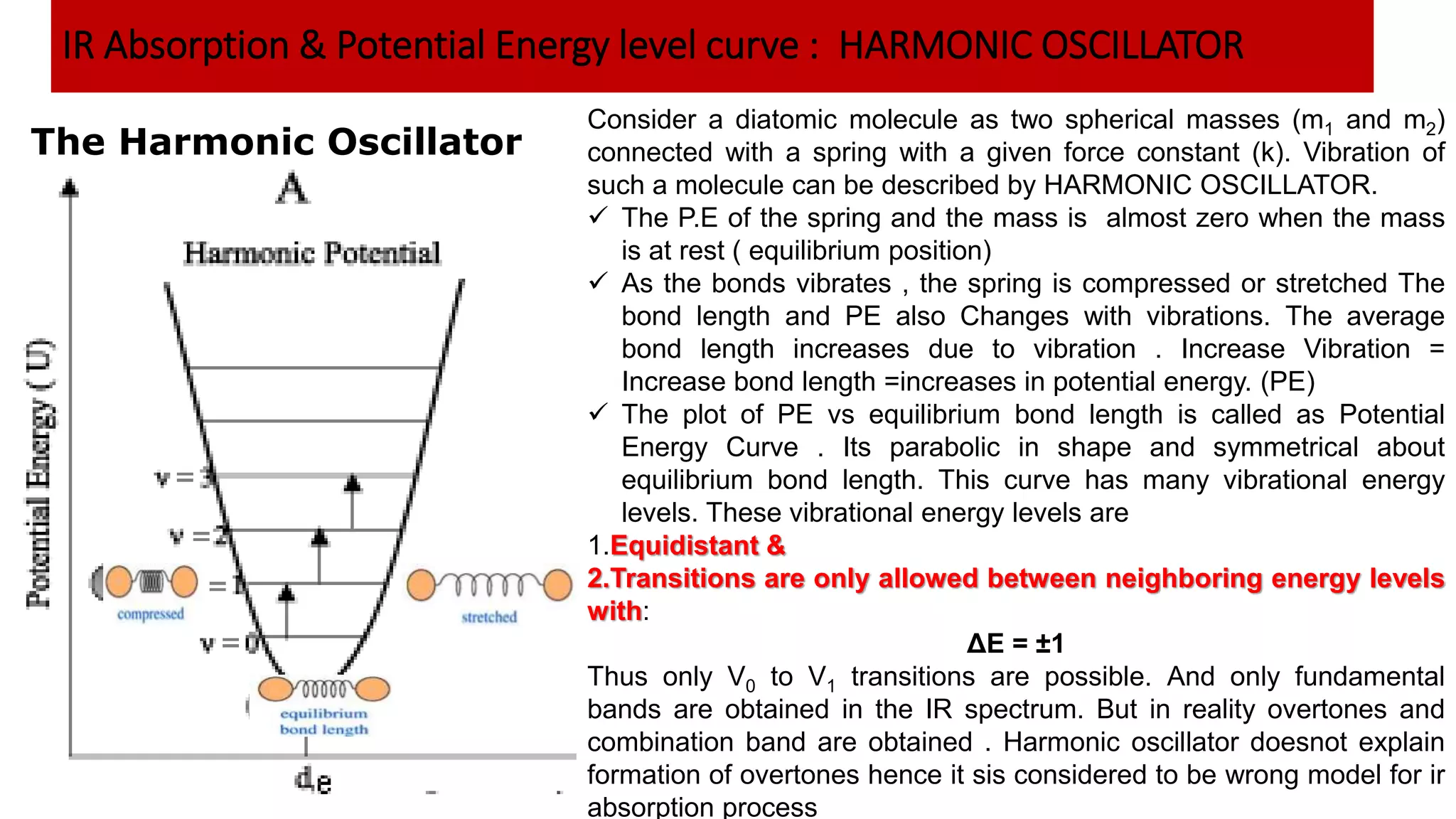
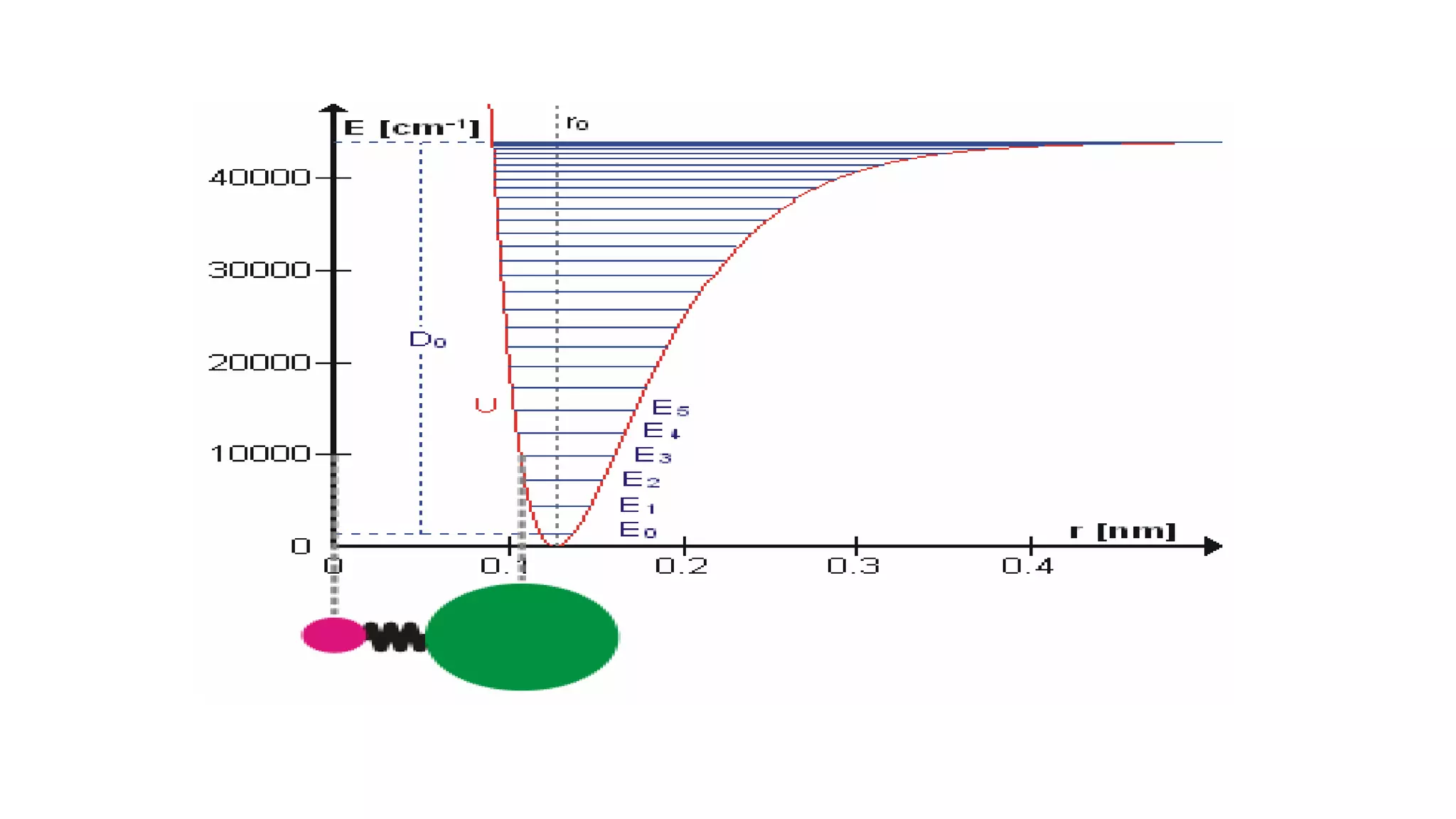
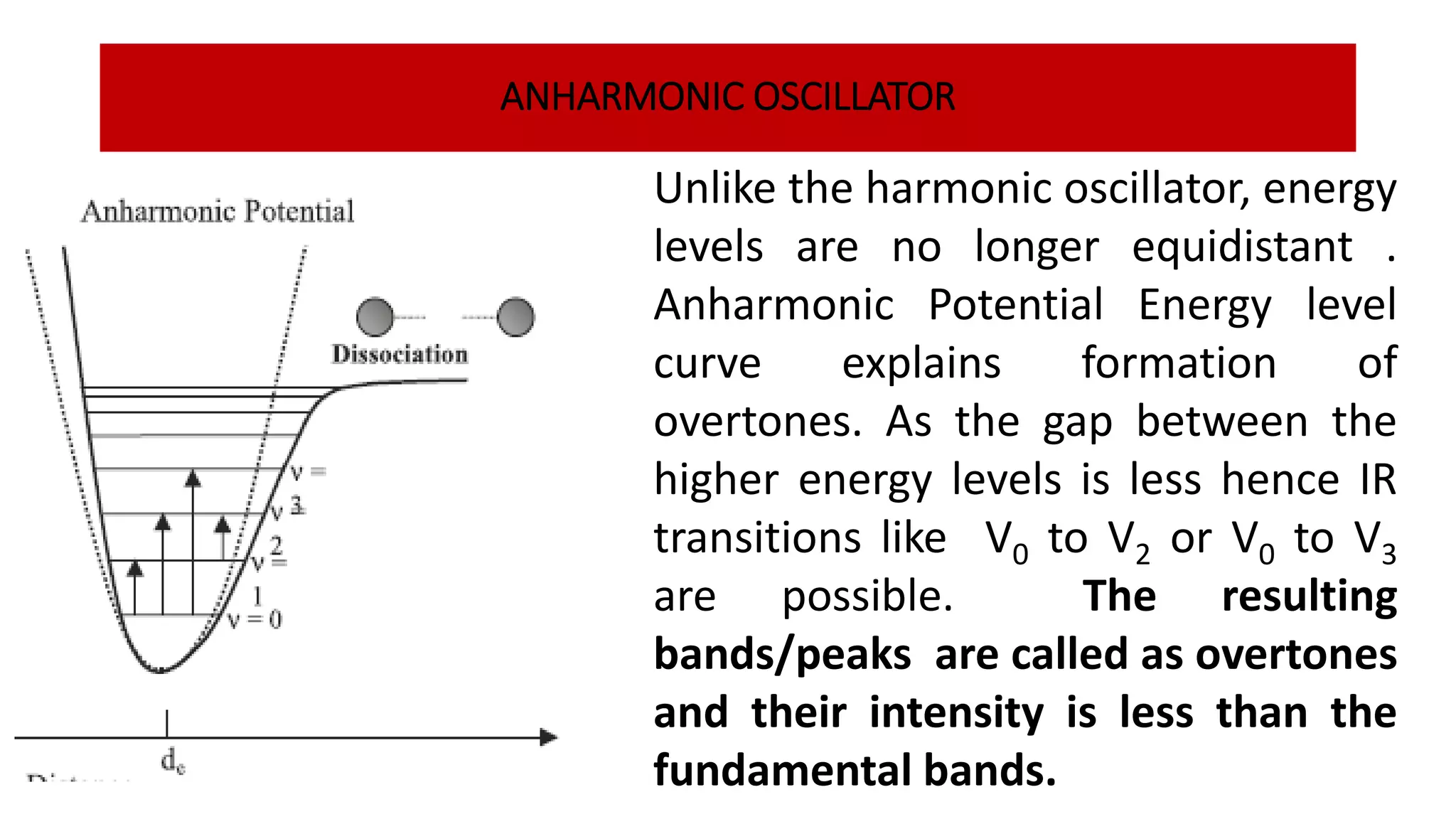
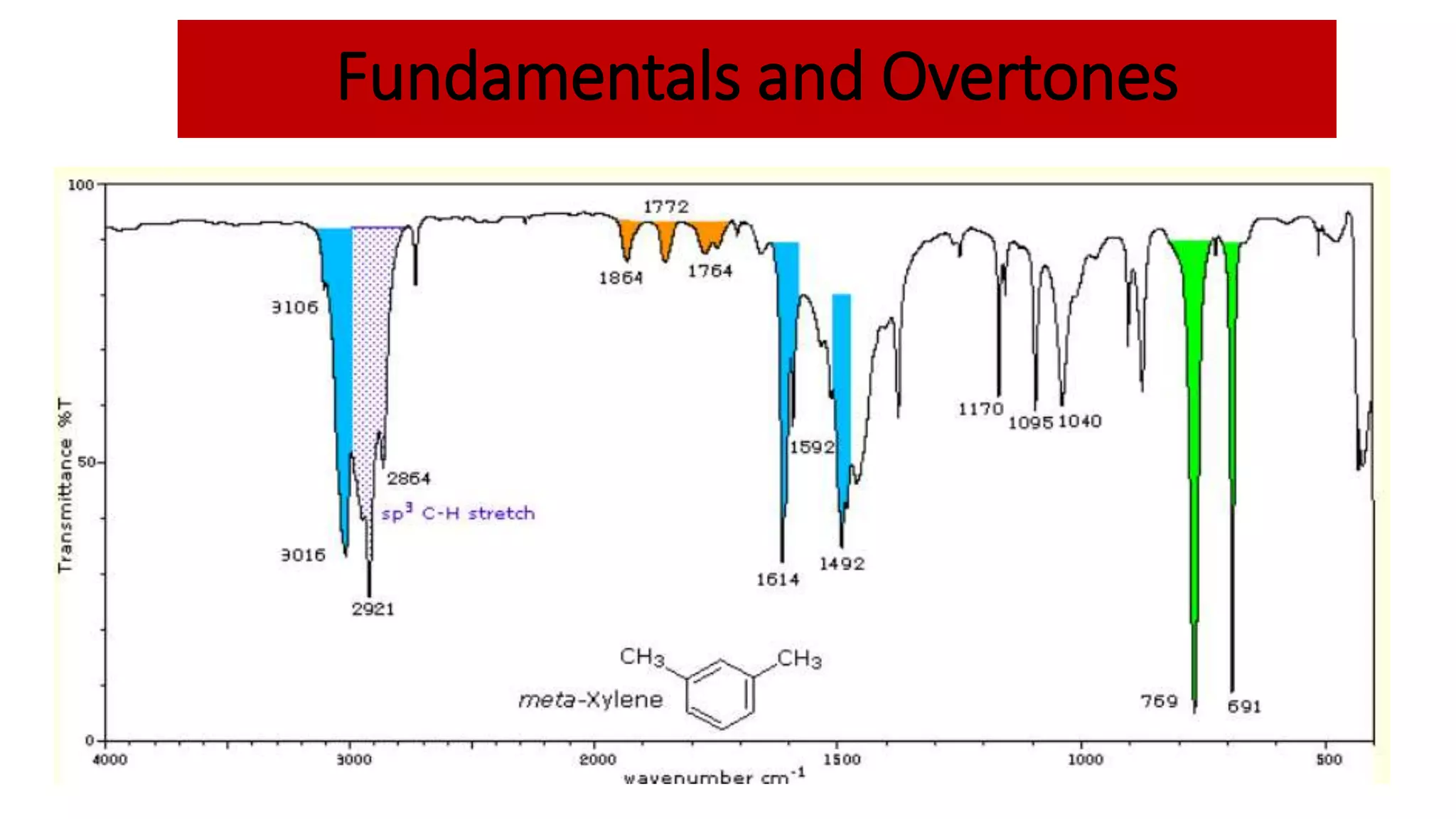
Infrared spectroscopy, also known as vibrational-rotational spectroscopy, involves the absorption of infrared radiation by molecules, leading to increased vibrational and rotational energy. It is used qualitatively to determine molecular structures and identify specific functional groups, with the spectrum acting as a chemical fingerprint unique to each molecule. The theory behind infrared absorption is rooted in molecular vibrations and the modification from harmonic to anharmonic oscillators, which explains the presence of overtones and combination bands in the spectra.