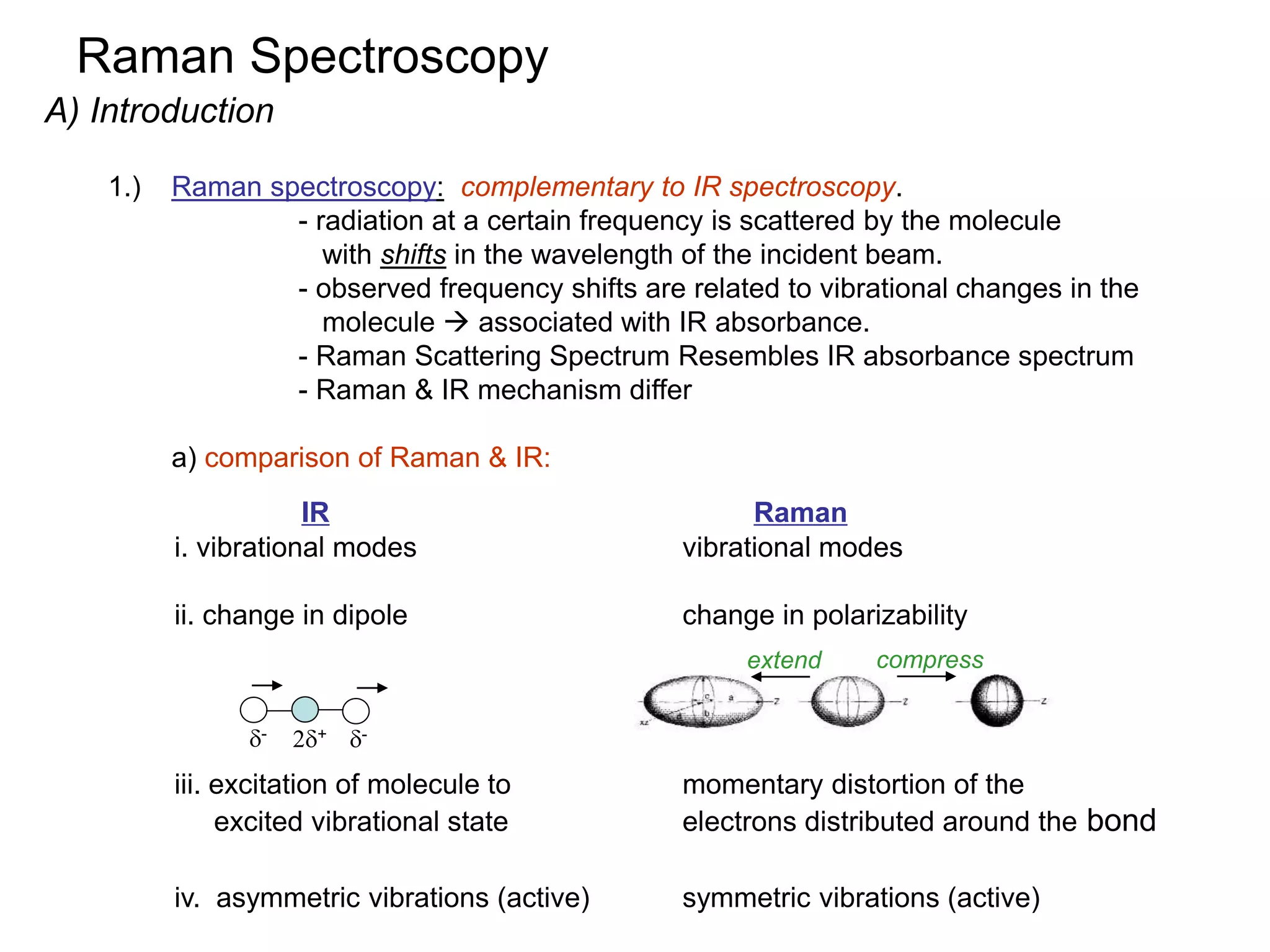
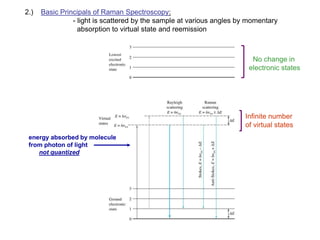
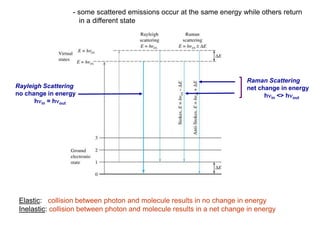
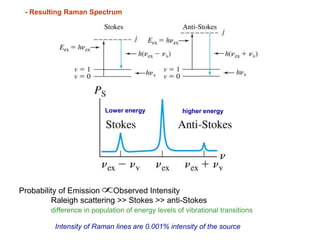
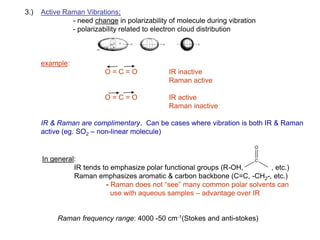
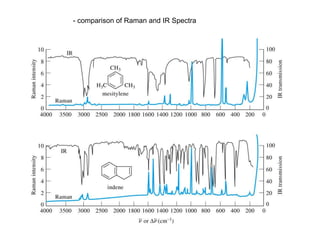
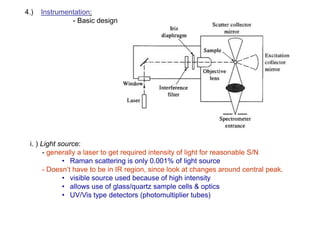
Raman spectroscopy is complementary to infrared spectroscopy. It involves scattering of monochromatic light, usually from a laser, with the frequency of photons in the scattered radiation shifted up or down relative to the incident photons. This shift provides information about vibrational modes in the molecule. Raman scattering arises from a change in polarizability rather than a change in dipole moment as in infrared spectroscopy. The Raman effect occurs when the laser light interacts with molecular vibrations, phonons or other excitations, resulting in the energy of the laser photons being shifted up or down. The shift in energy allows the measurement of vibrational modes in a system. Raman spectroscopy is a useful technique for qualitative and quantitative analysis of organic, inorganic, and biological samples