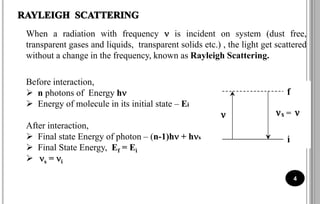
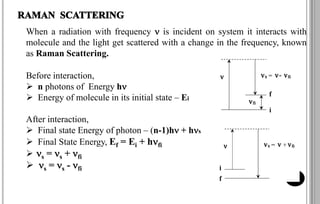
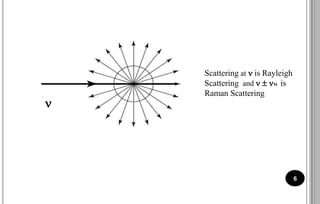
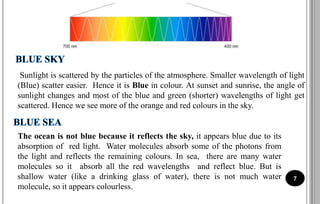
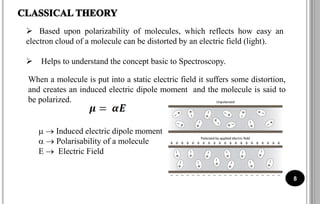
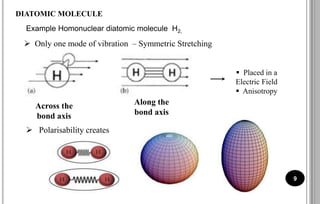
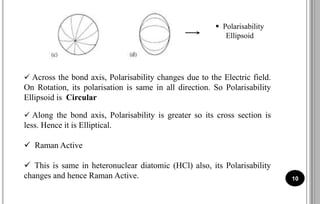
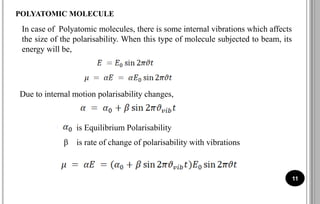
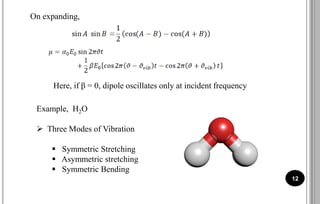
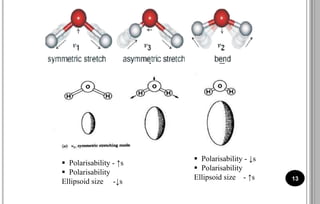
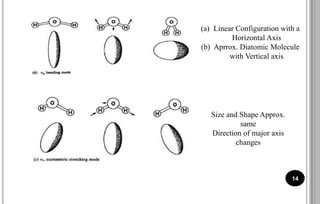
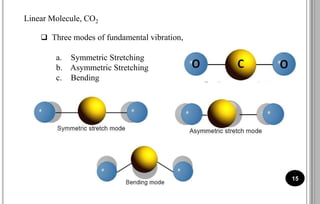
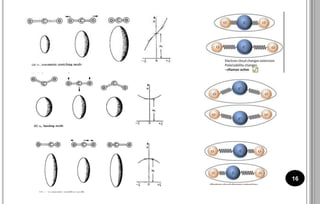
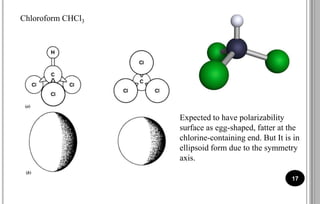
The document discusses various scattering phenomena related to light, focusing on Rayleigh and Raman scattering. It explains why the sky appears blue due to the scattering of shorter wavelengths of light and how the ocean appears blue due to its absorption of red light. Additionally, the document covers molecular polarisability and its effects on Raman activity in both diatomic and polyatomic molecules.