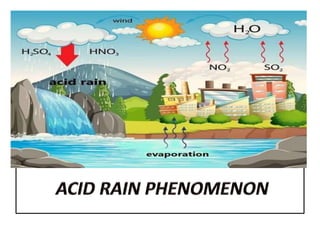
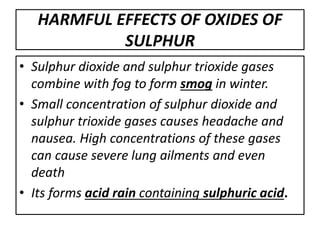
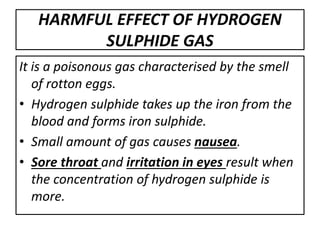
This document summarizes the key points about acid rain. It defines acid rain as rain containing nitric acid and sulfuric acid formed when gases like sulfur dioxide and nitrogen dioxide dissolve in water. These gases are released from the combustion of fossil fuels and other industrial processes. Acid rain lowers the pH of lakes and soils, damaging plants and aquatic life. It also corrodes infrastructure and erodes marble structures like the Taj Mahal. The document recommends reducing gas emissions from industries and using pollution control devices to mitigate acid rain.