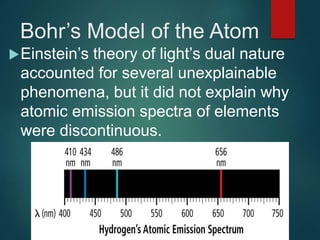
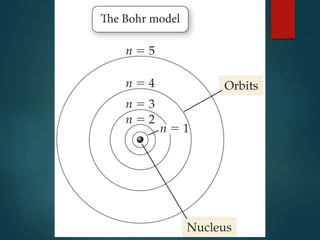
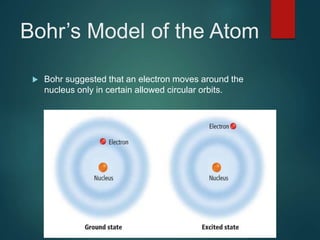
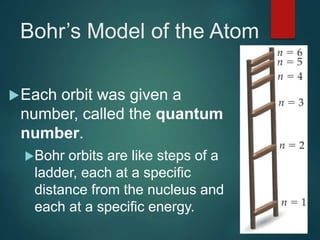
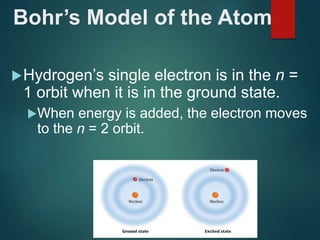
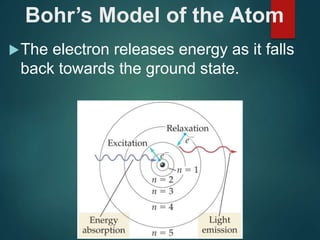
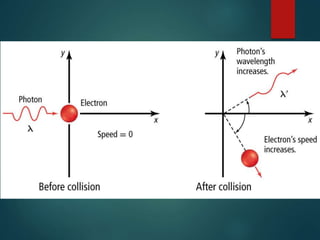
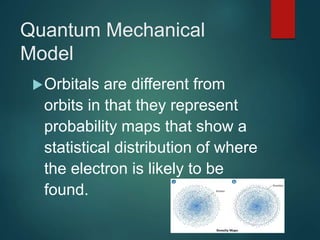
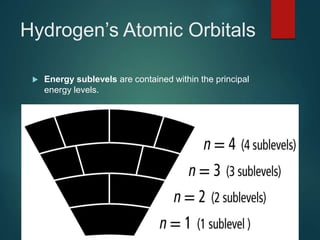
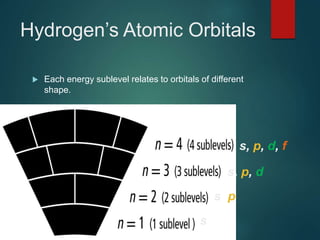
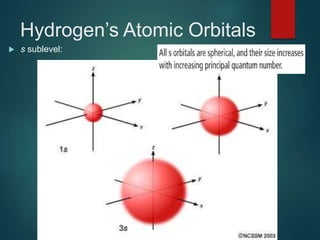
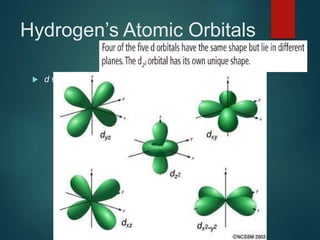
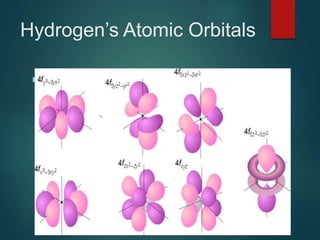
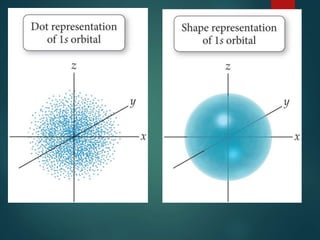
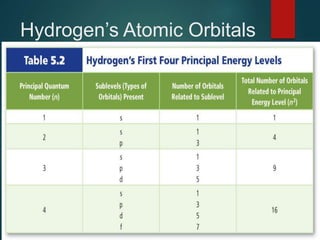
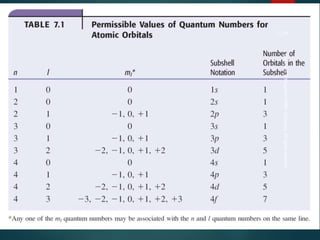
The document discusses the quantum mechanical model of the atom. It explains how the Bohr model was replaced by the quantum mechanical model based on the work of scientists like de Broglie, Heisenberg, and Schrodinger. The quantum mechanical model describes electrons as waves occupying atomic orbitals defined by quantum numbers rather than following precise orbits. The document outlines the key concepts of the quantum mechanical model including the principal, azimuthal, magnetic, and spin quantum numbers and how they define an electron's location and properties in an atom.