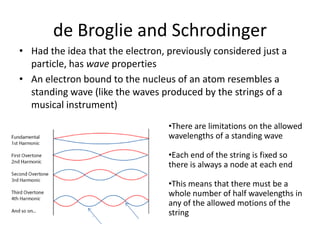
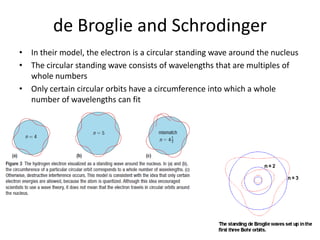
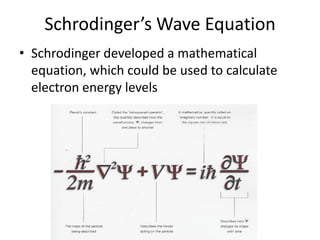
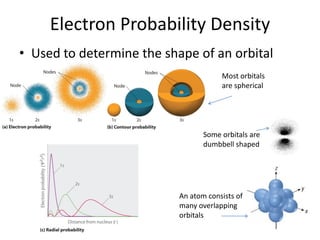
The quantum mechanical model of the atom developed from the ideas of three physicists: Erwin Schrodinger, Louis de Broglie, and Werner Heisenberg. De Broglie and Schrodinger proposed that electrons have both particle and wave properties, resembling standing waves around the nucleus. Only certain orbits allow an integer number of wavelengths to fit, determining electron energy levels. Schrodinger developed an equation to calculate these quantized energy levels. Heisenberg's uncertainty principle holds that the exact position and momentum of an electron cannot be known simultaneously. Orbitals describe the probability of finding an electron in a region as determined by wave functions, rather than definite orbits.