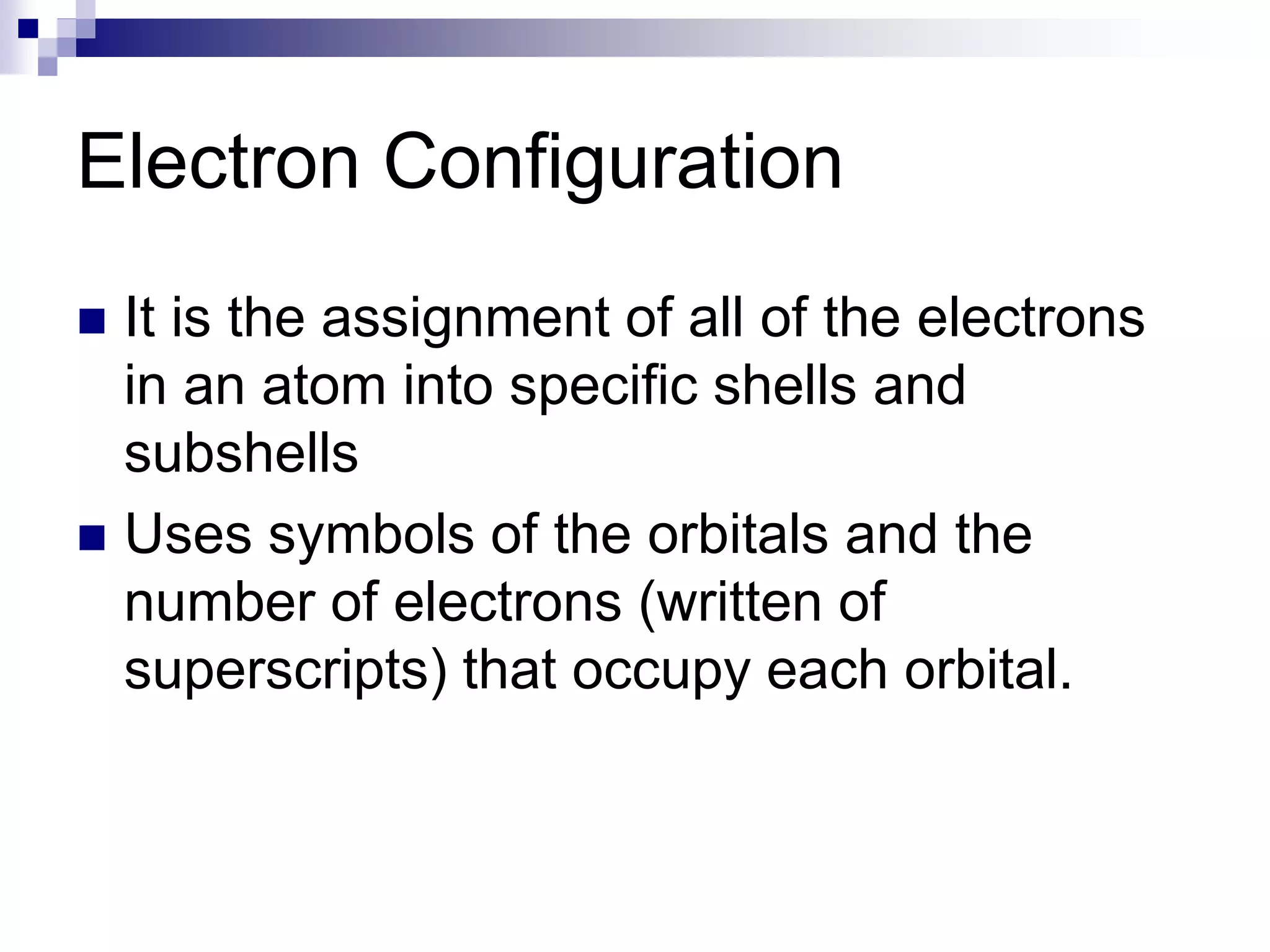
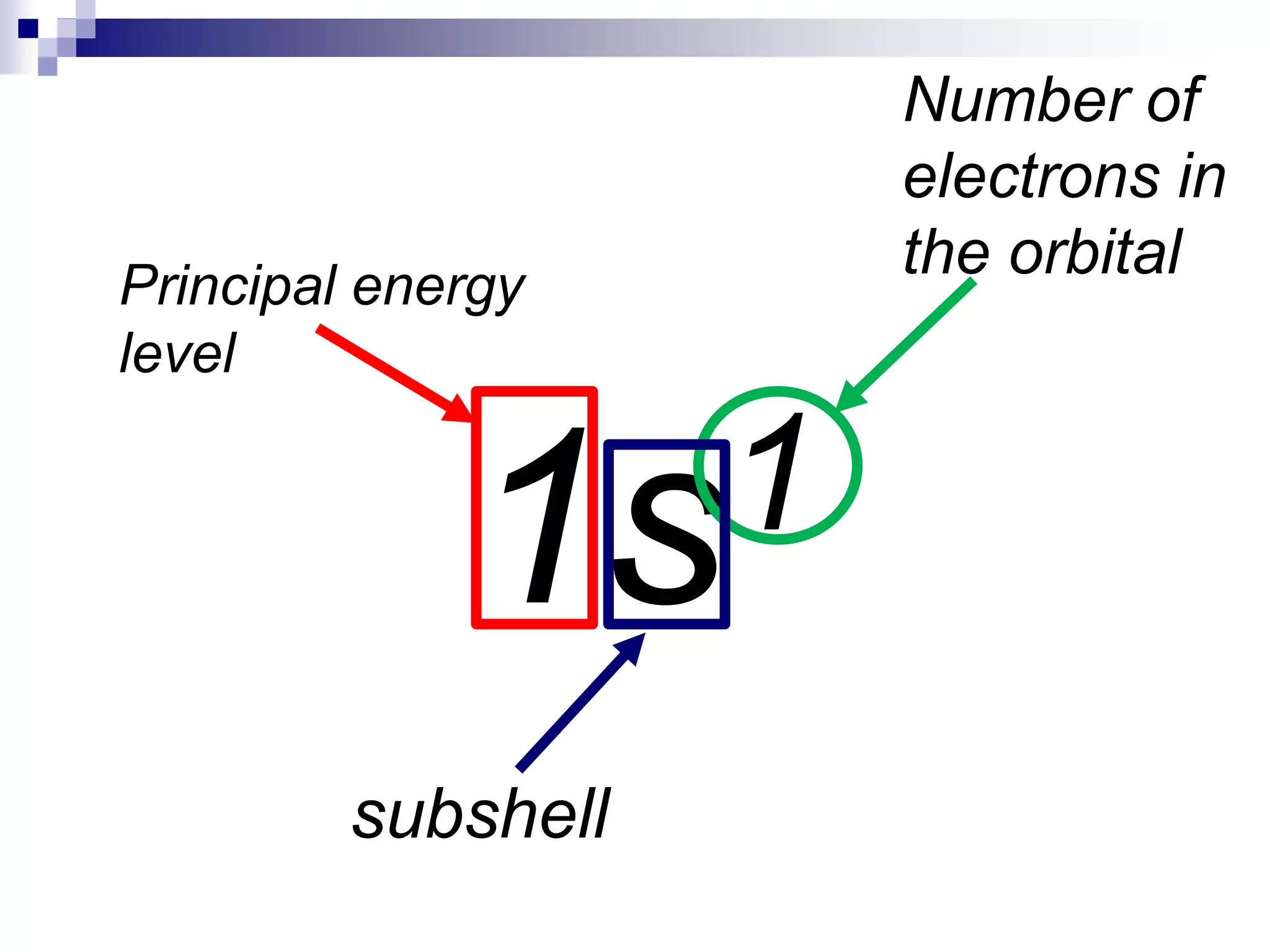
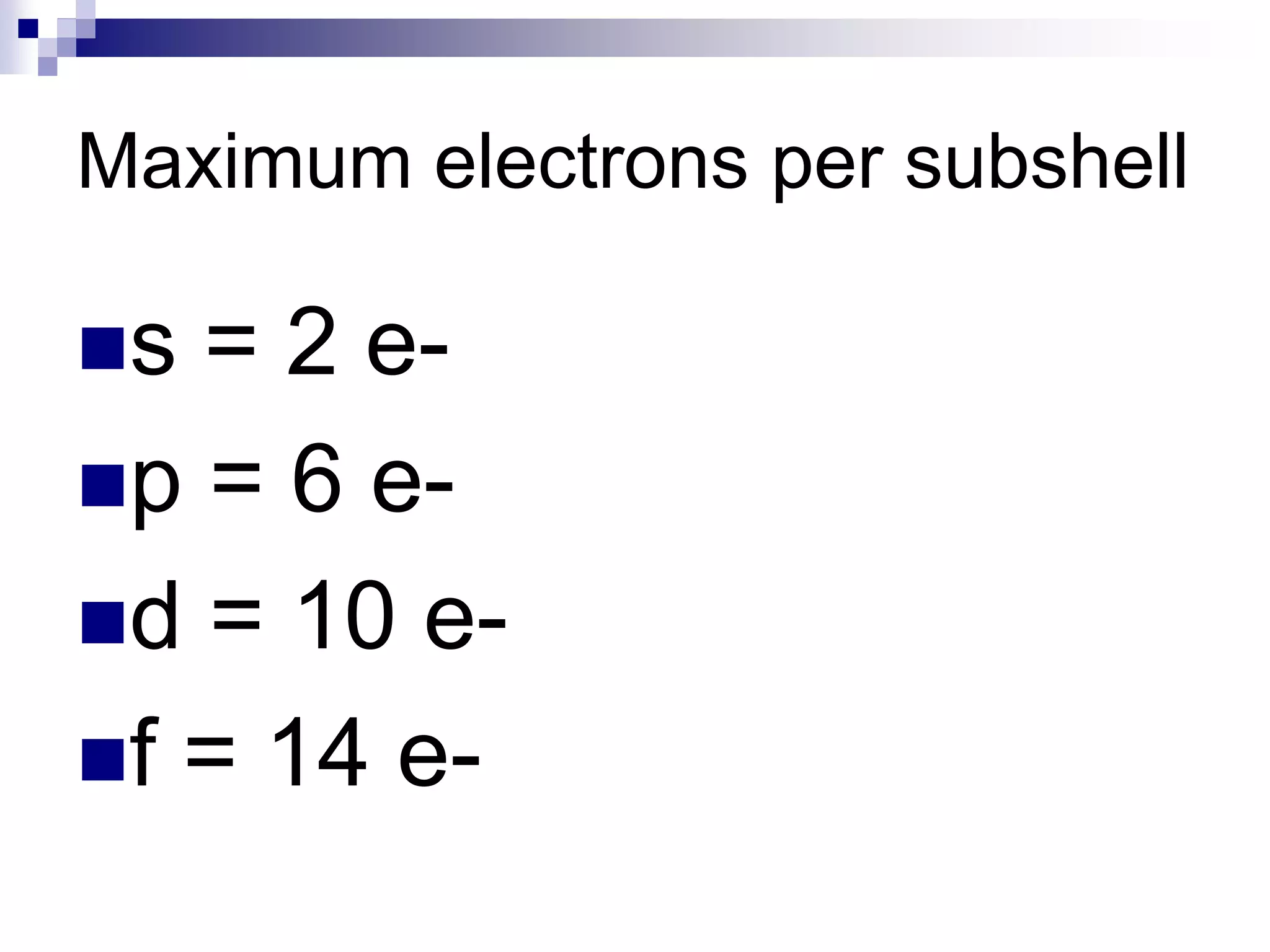
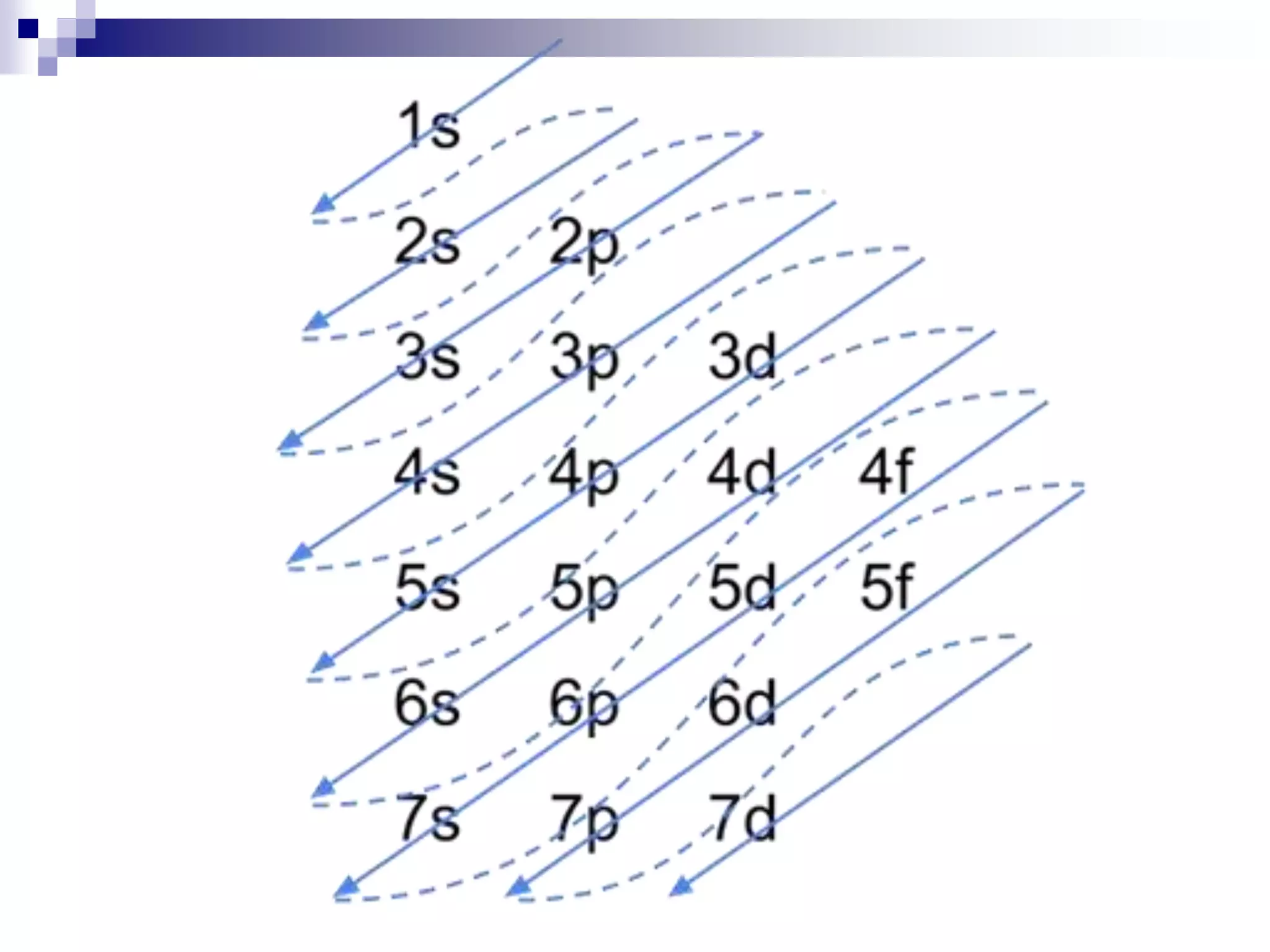
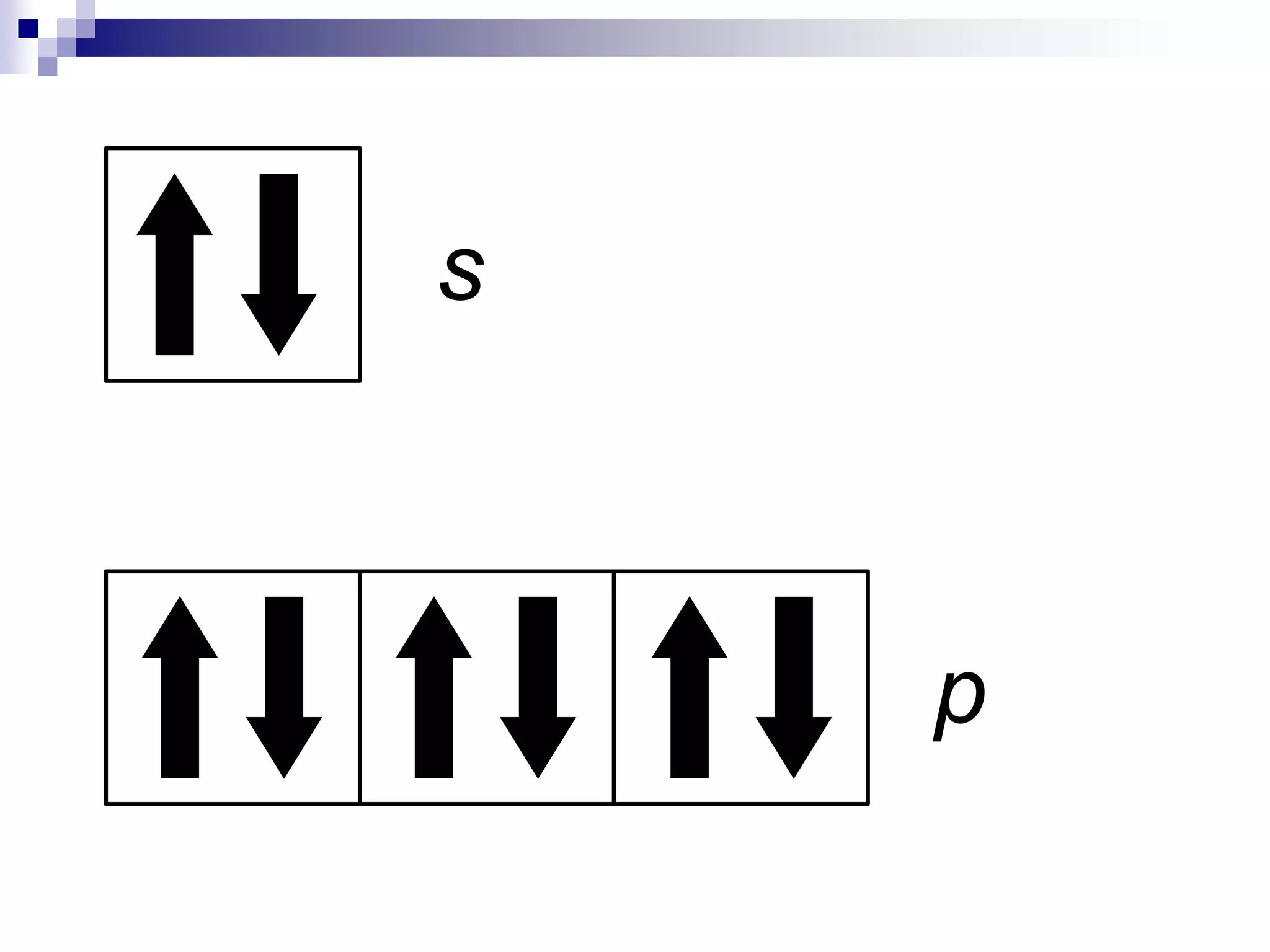
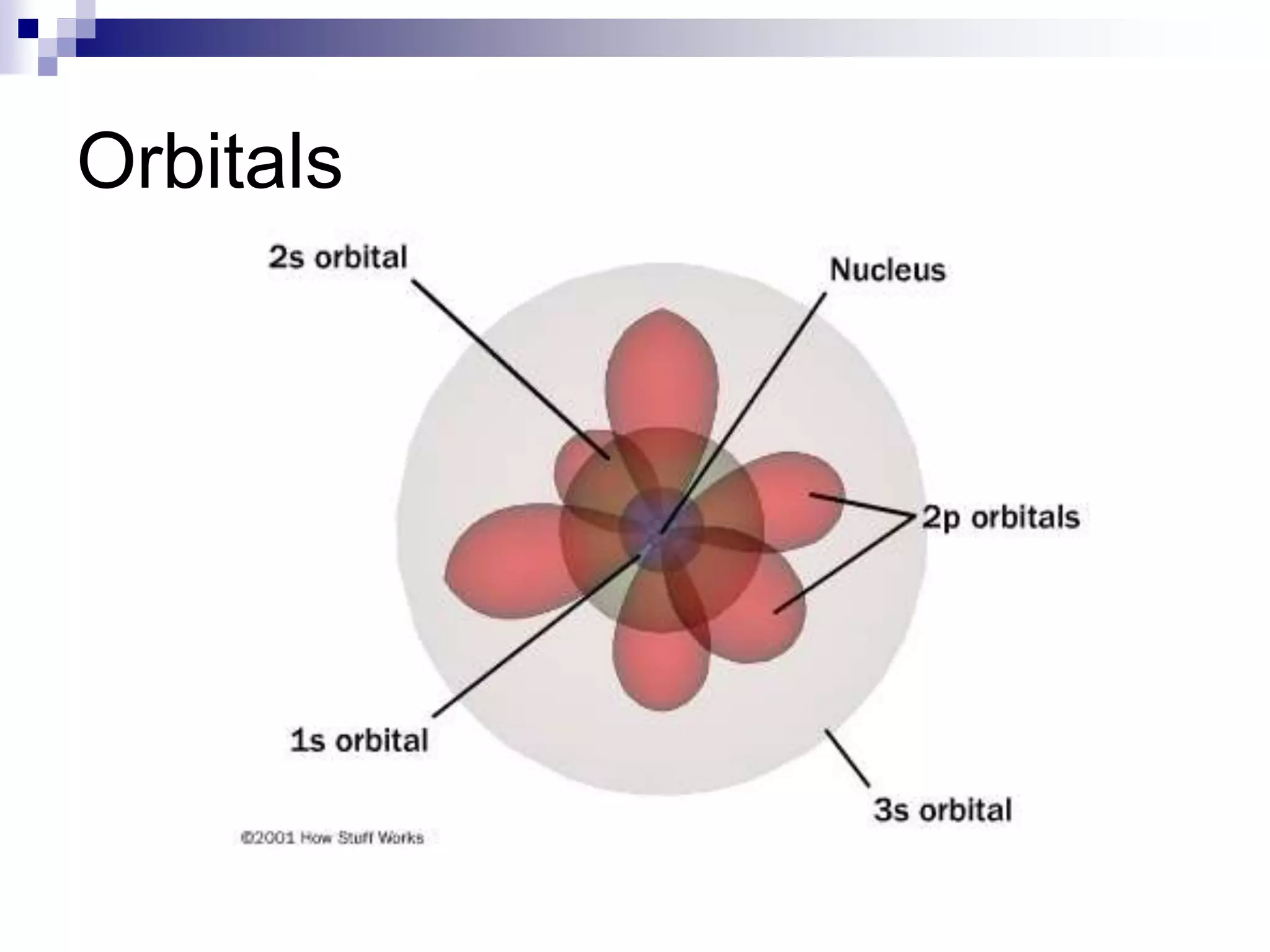
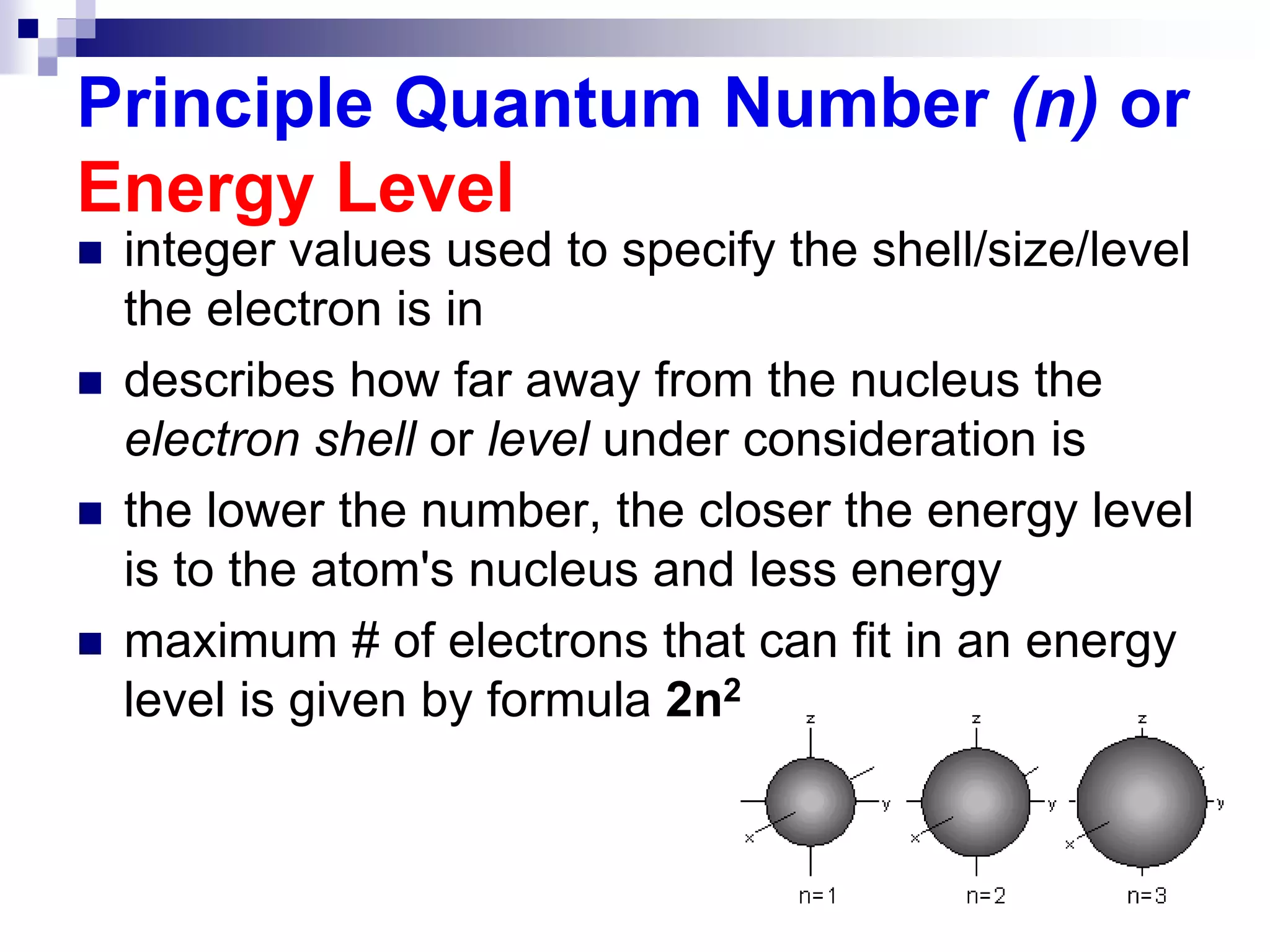
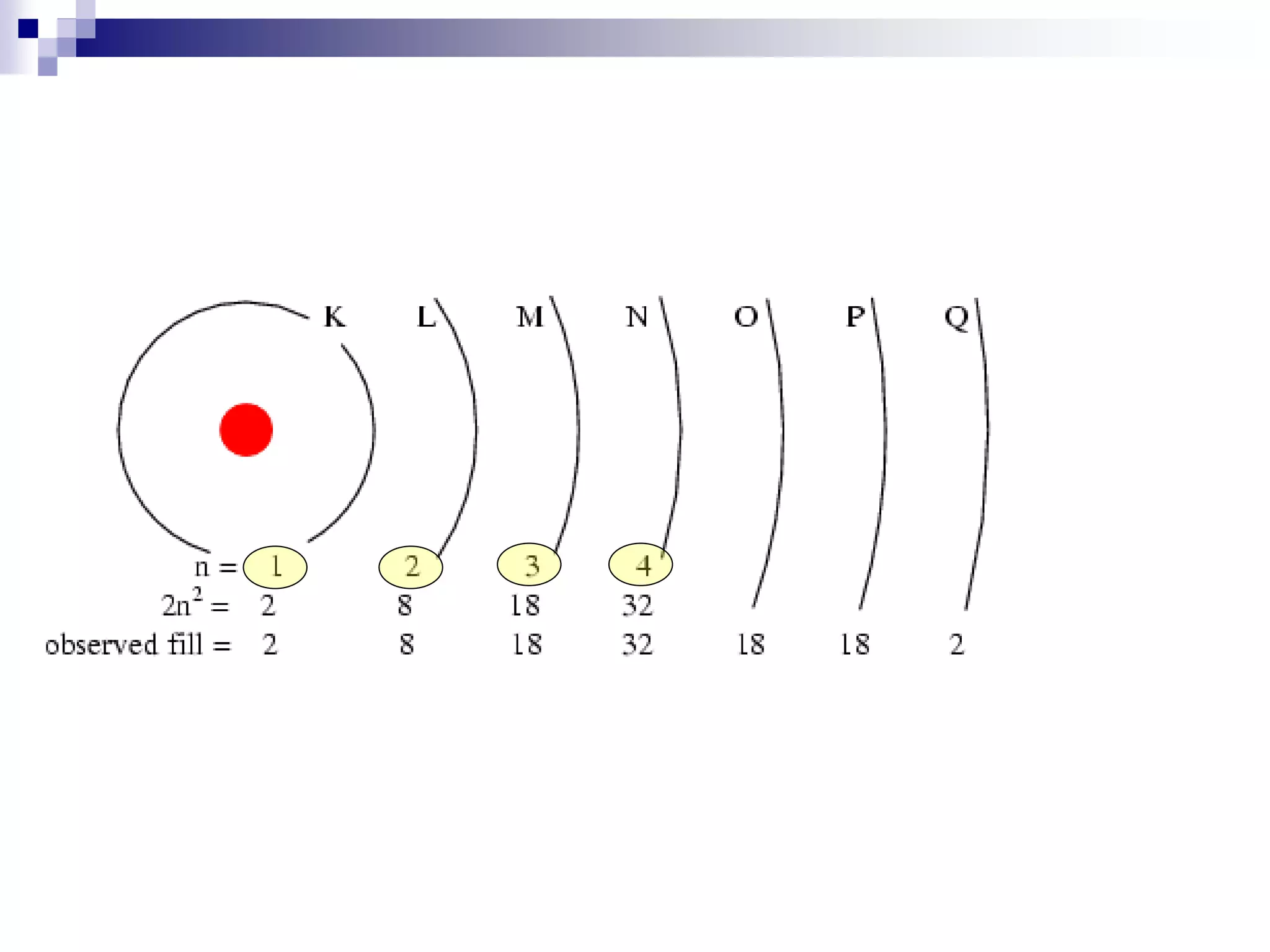
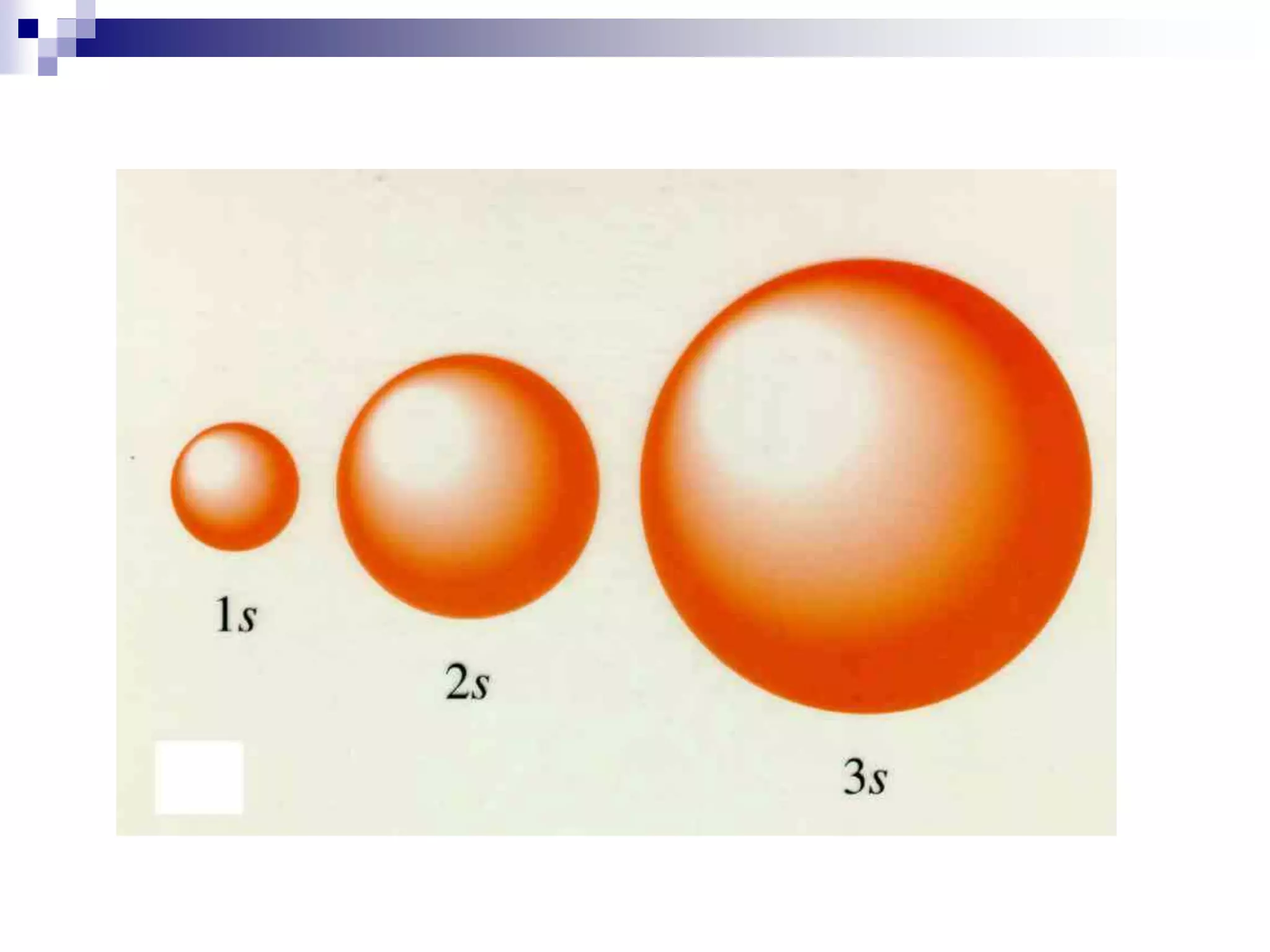
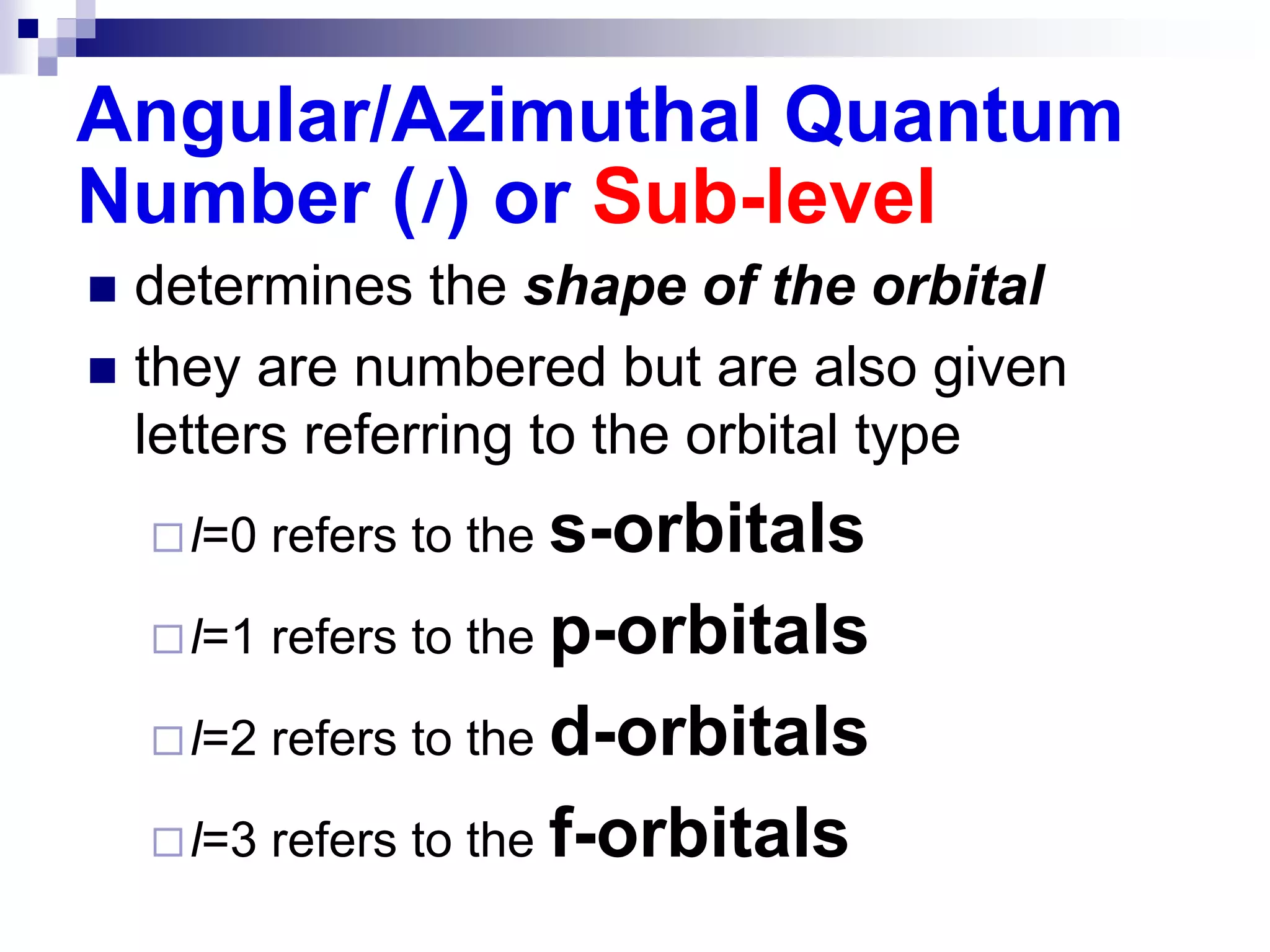
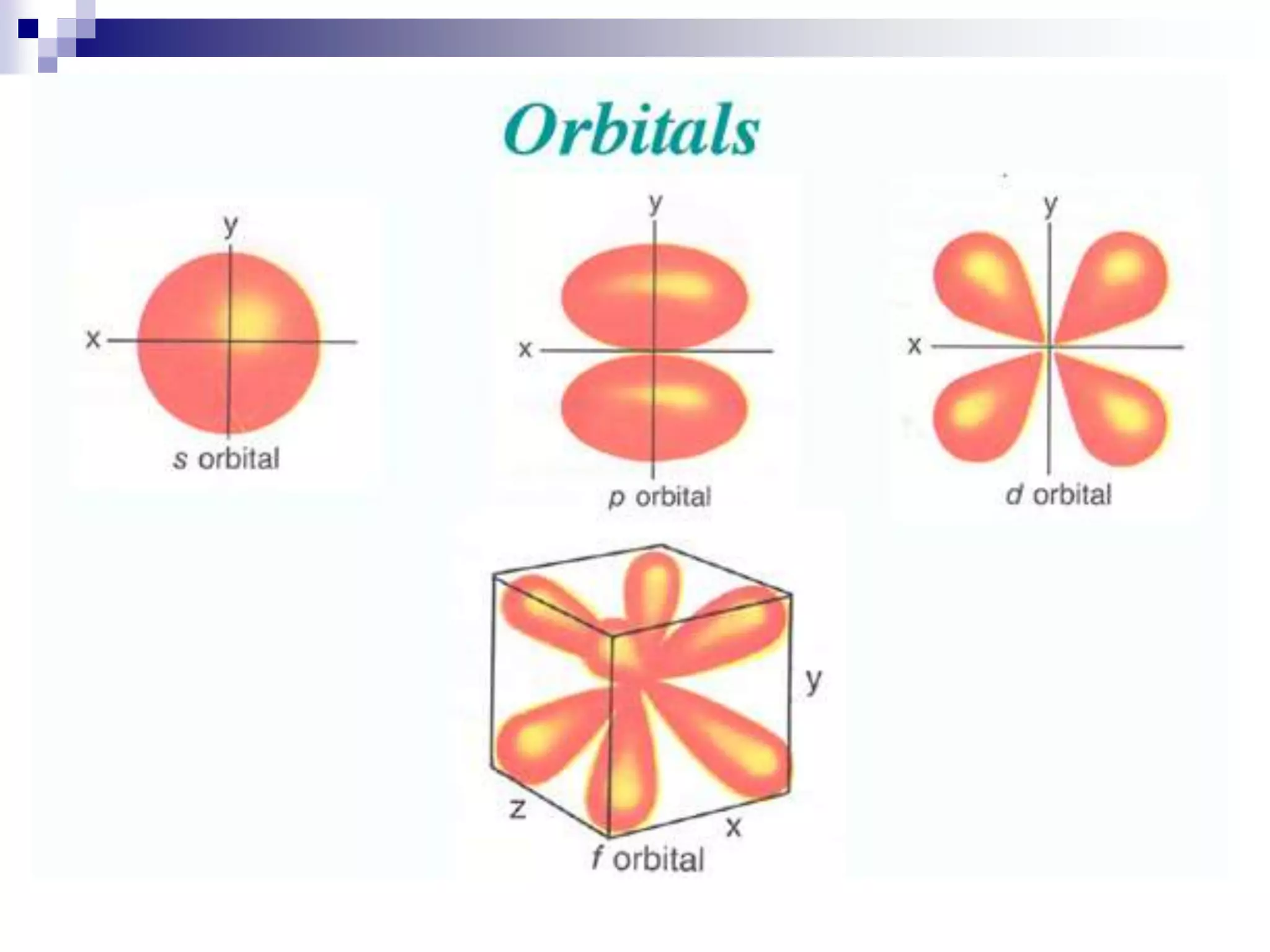
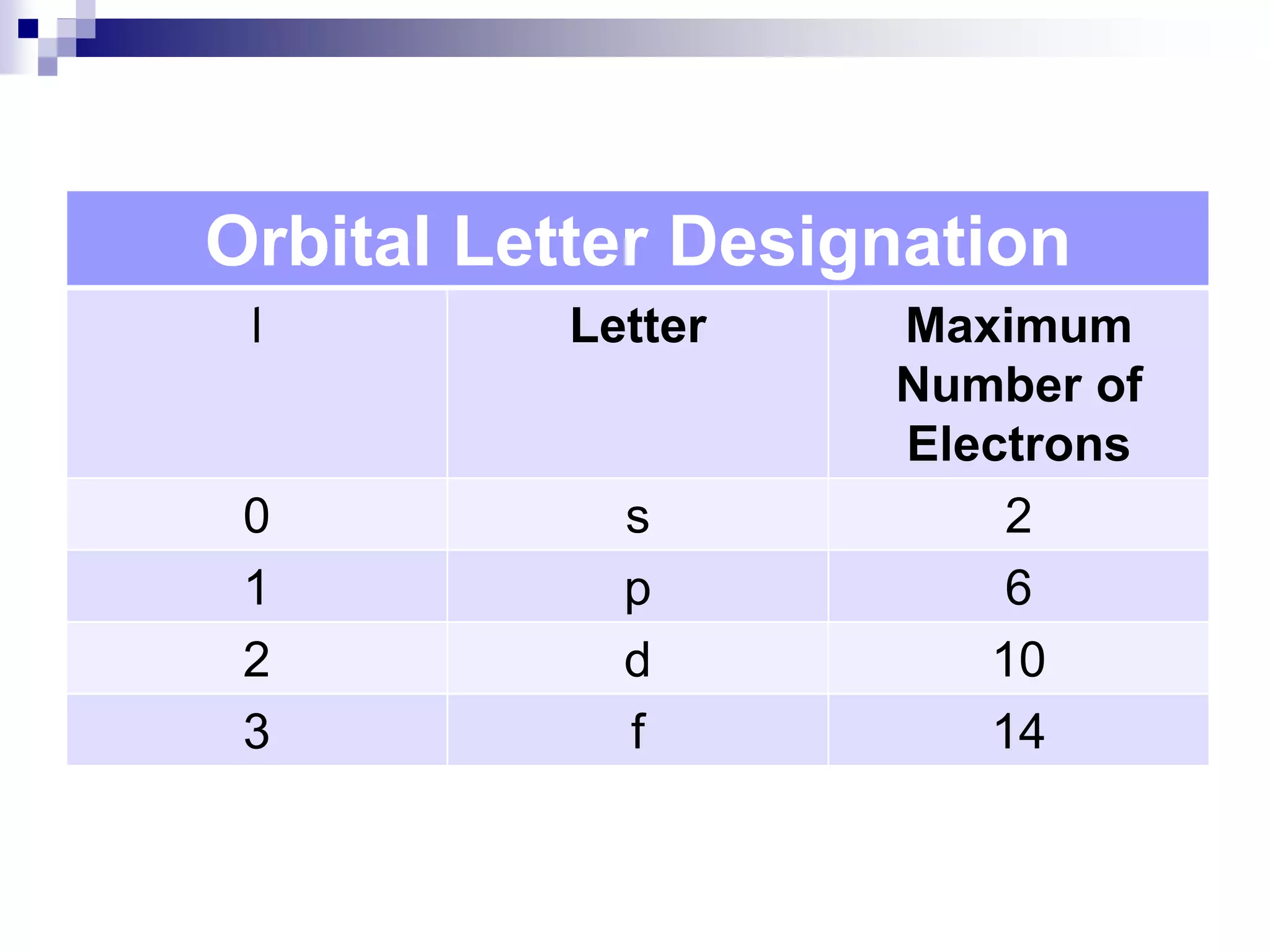
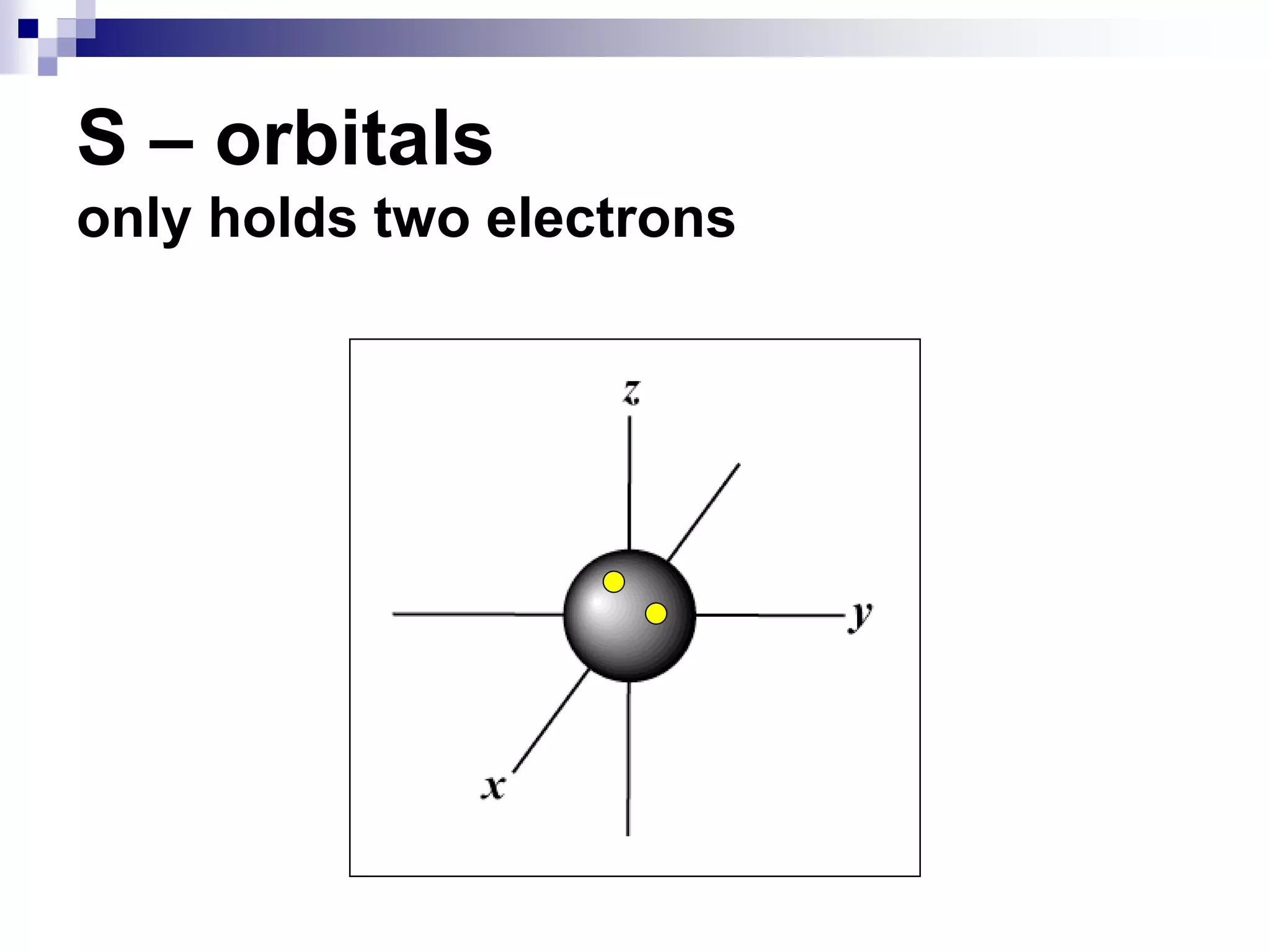
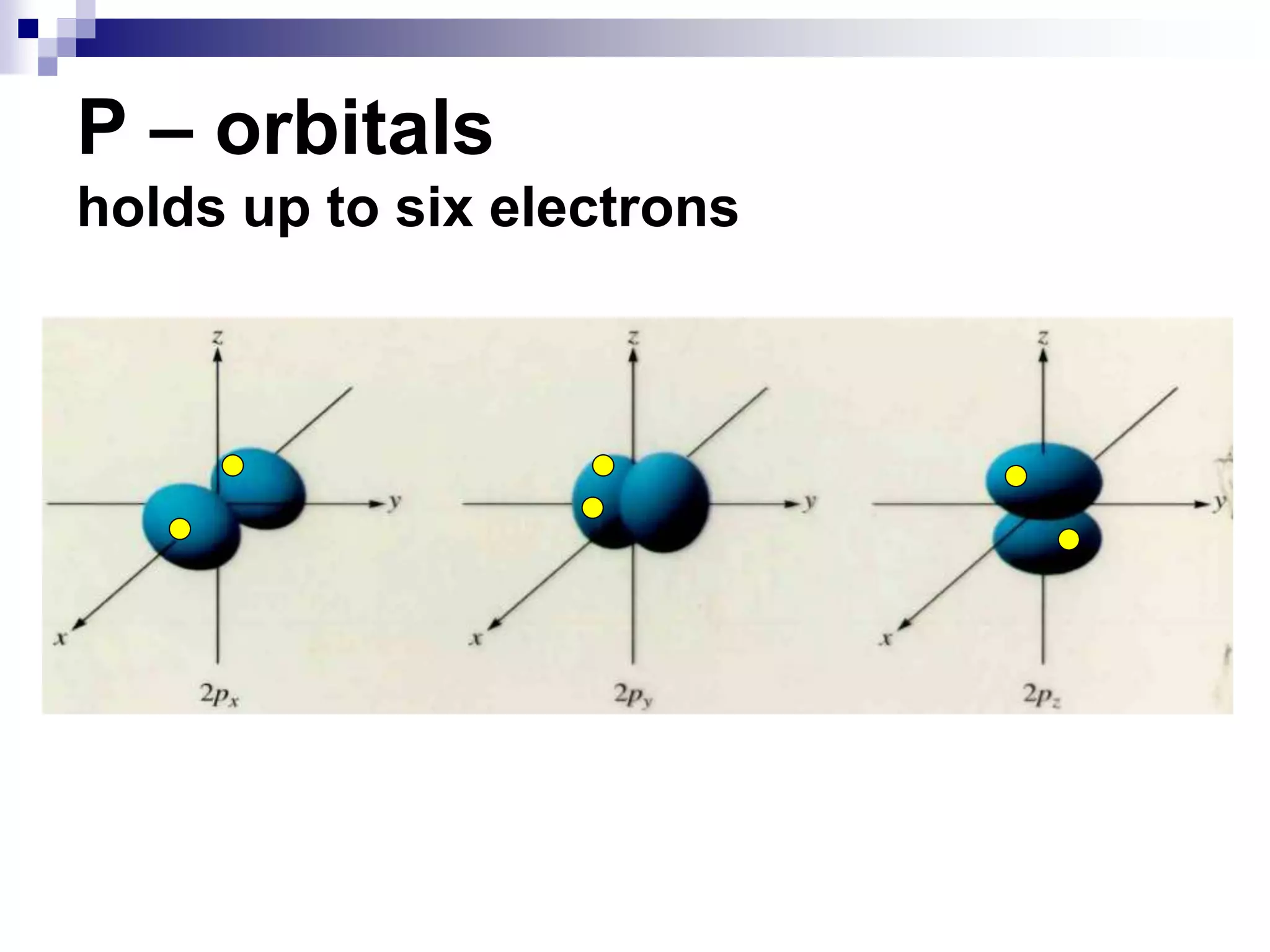
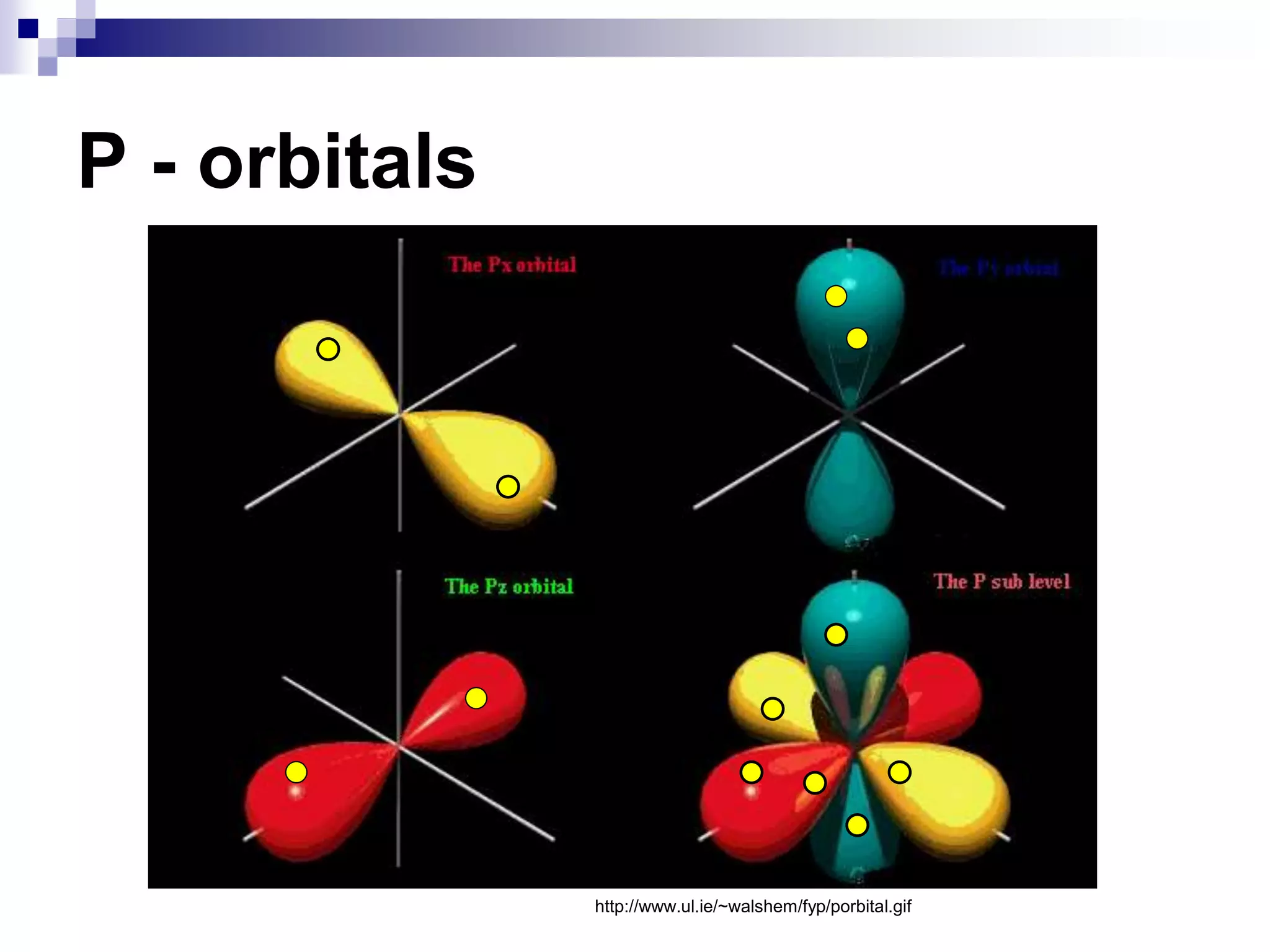
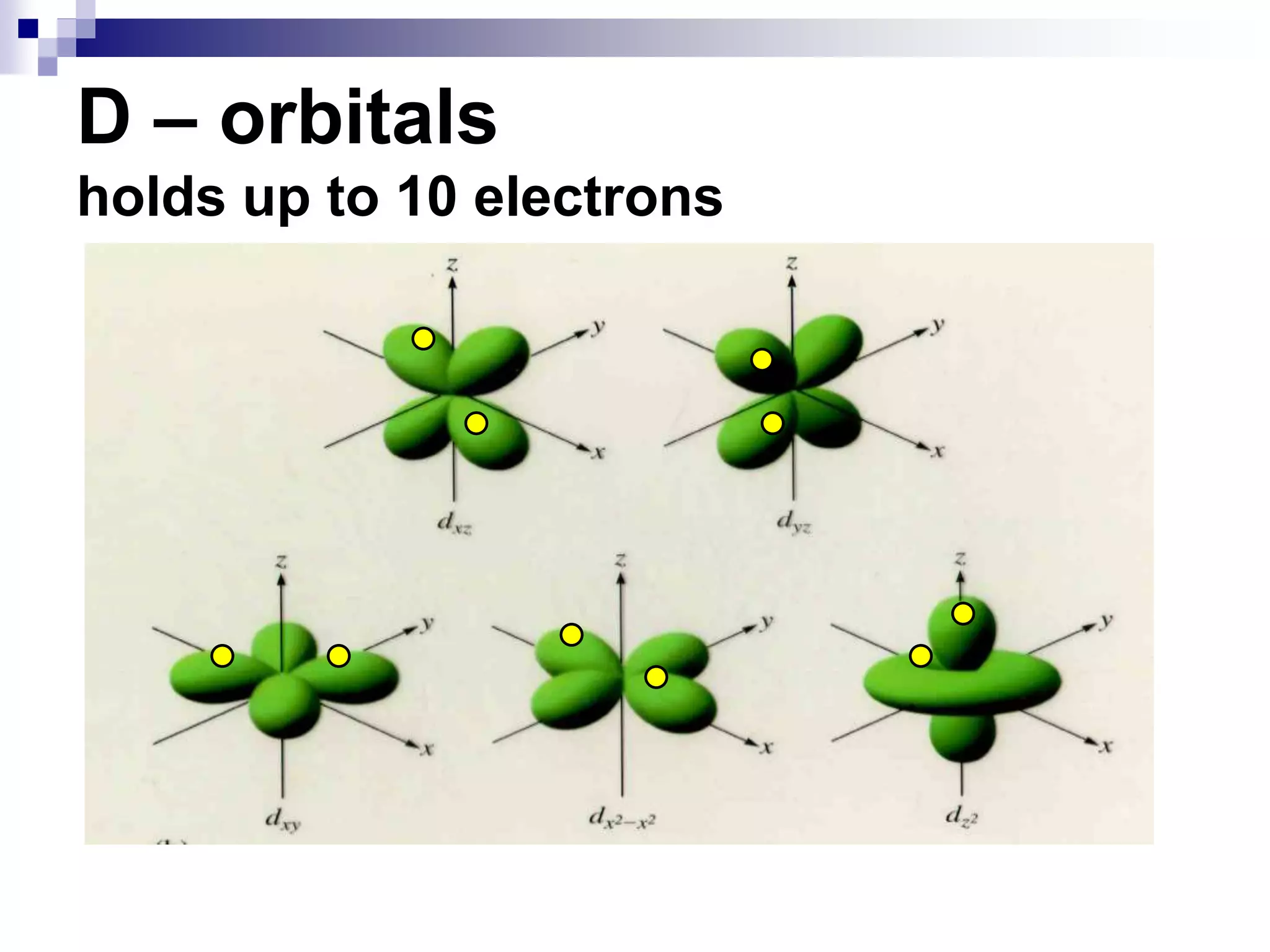
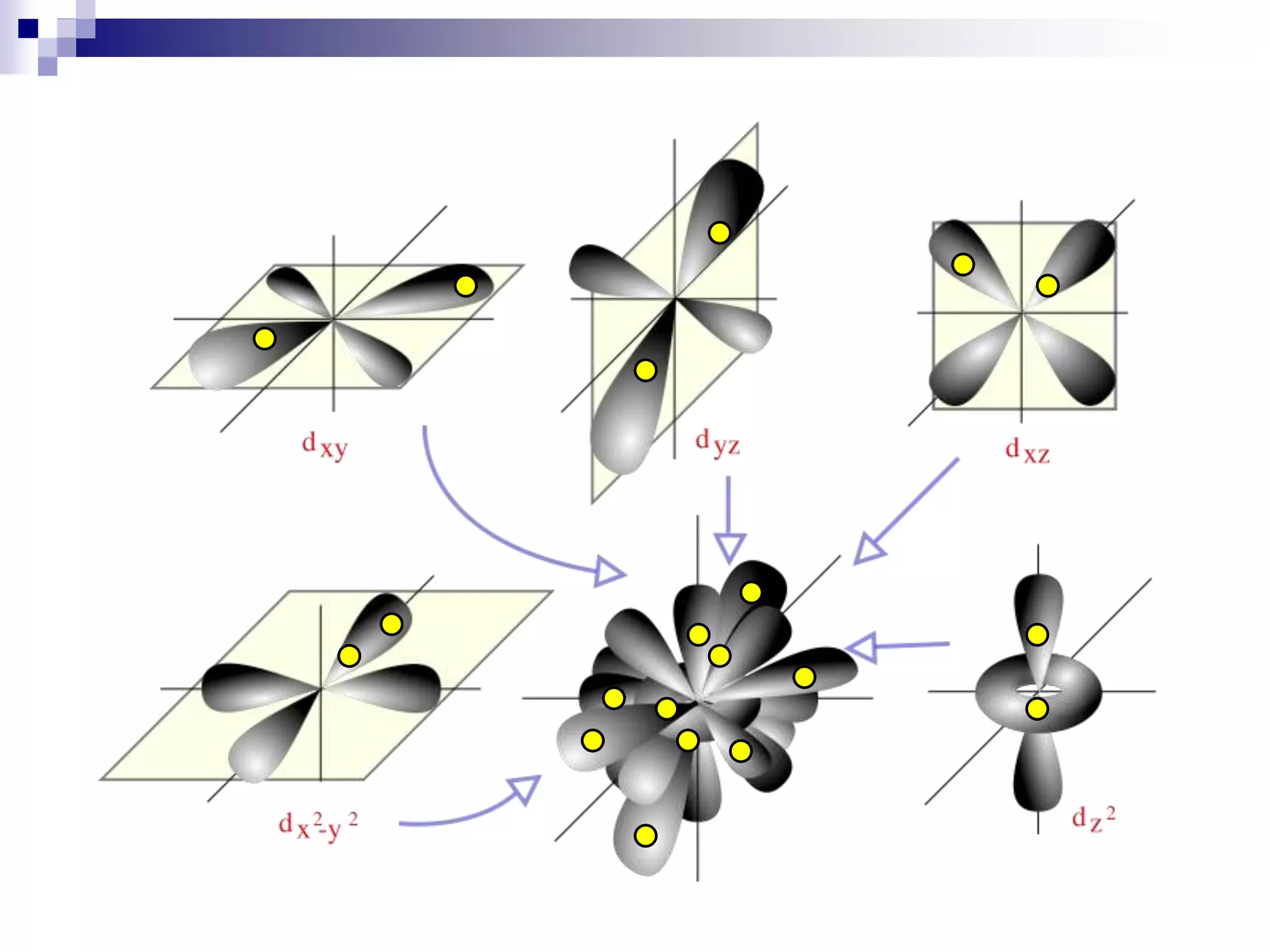
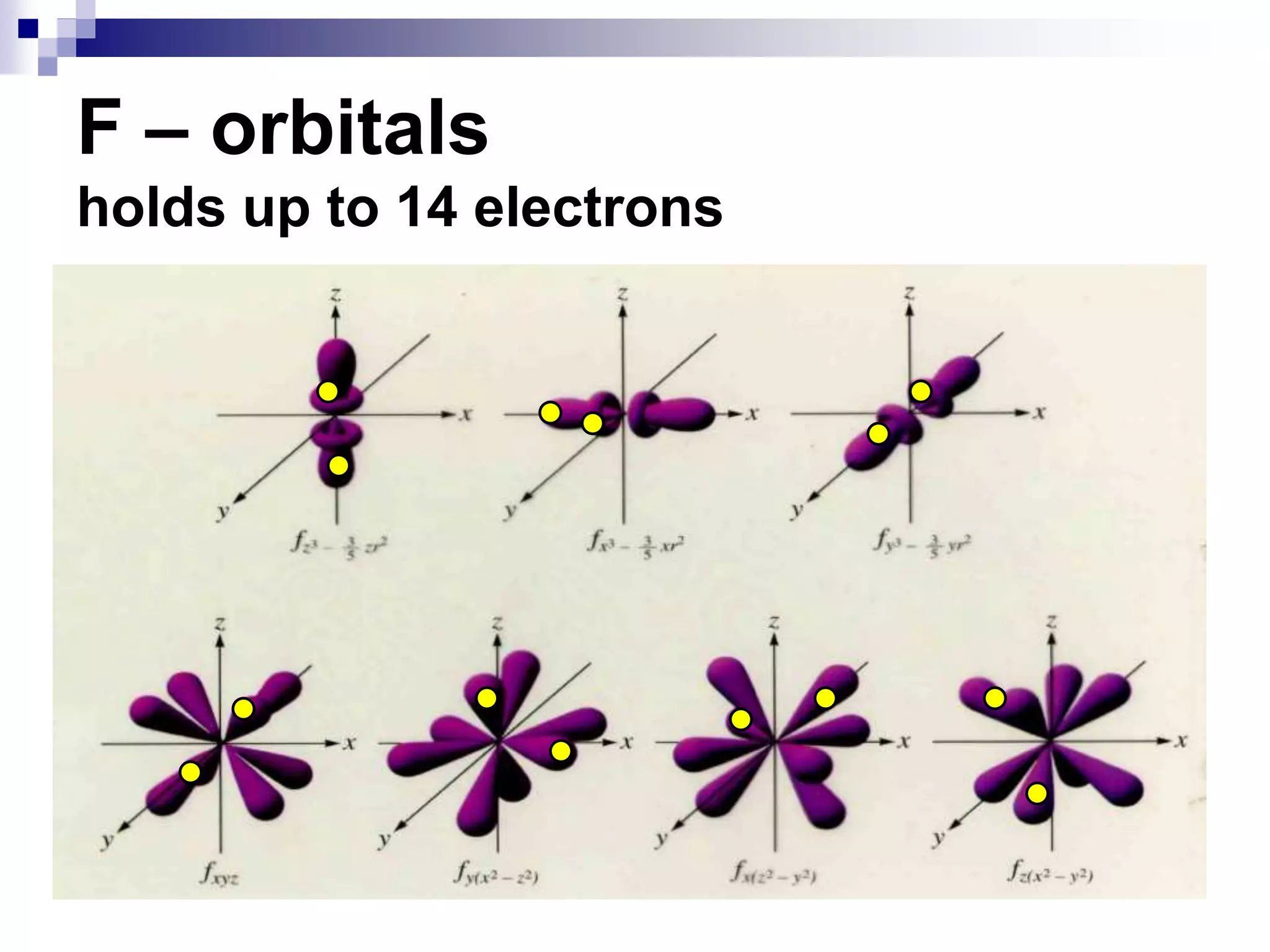
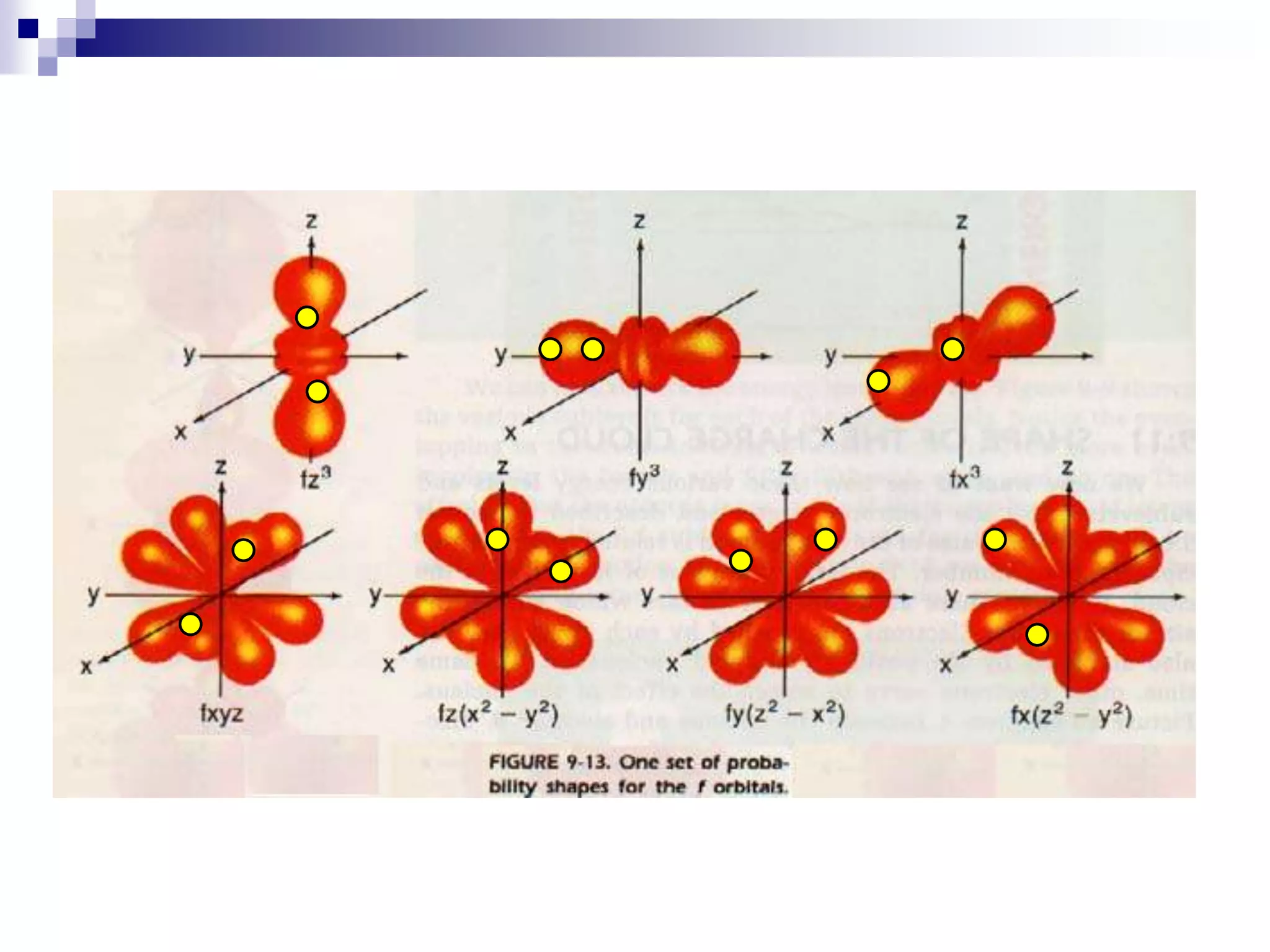
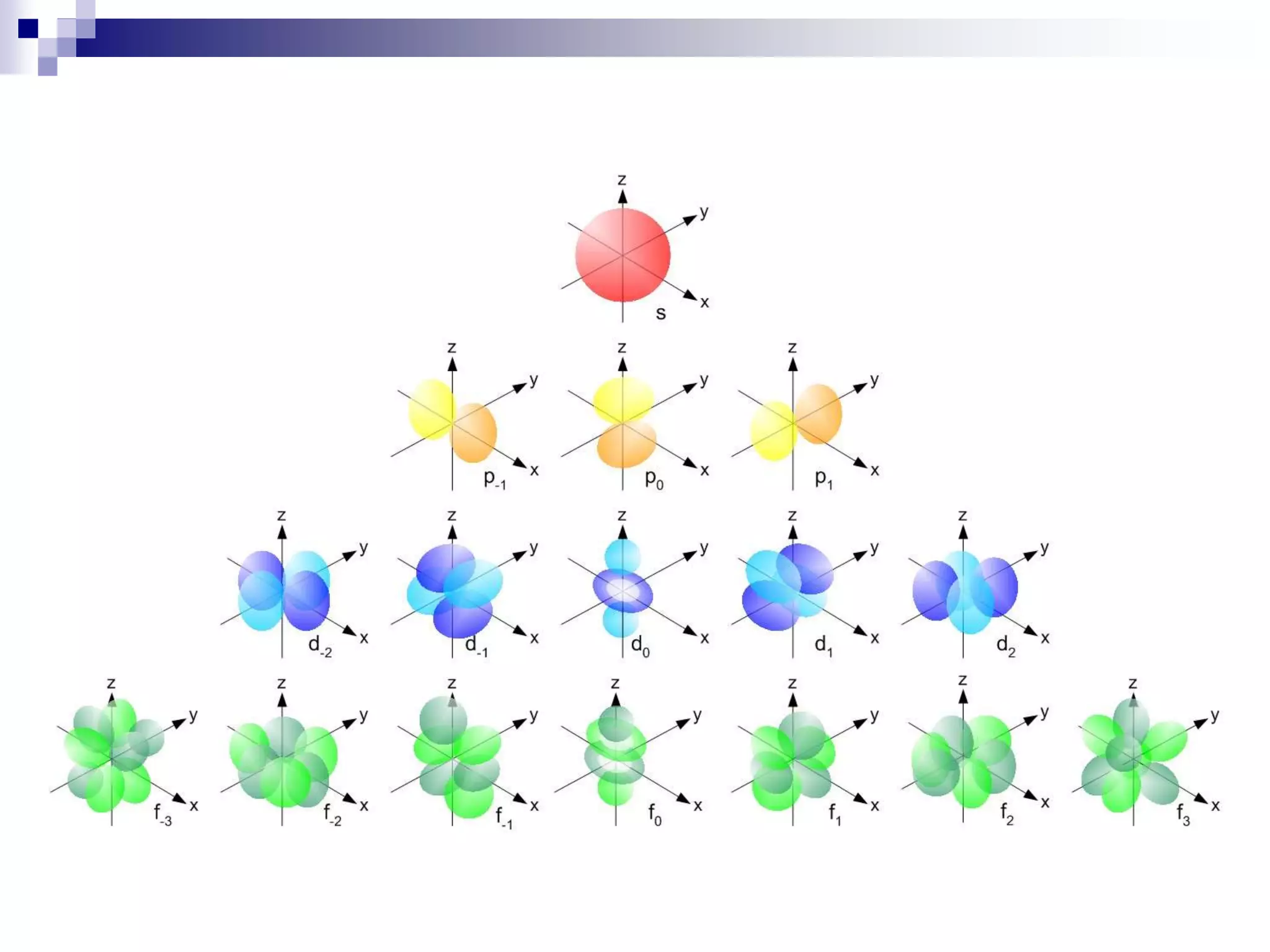
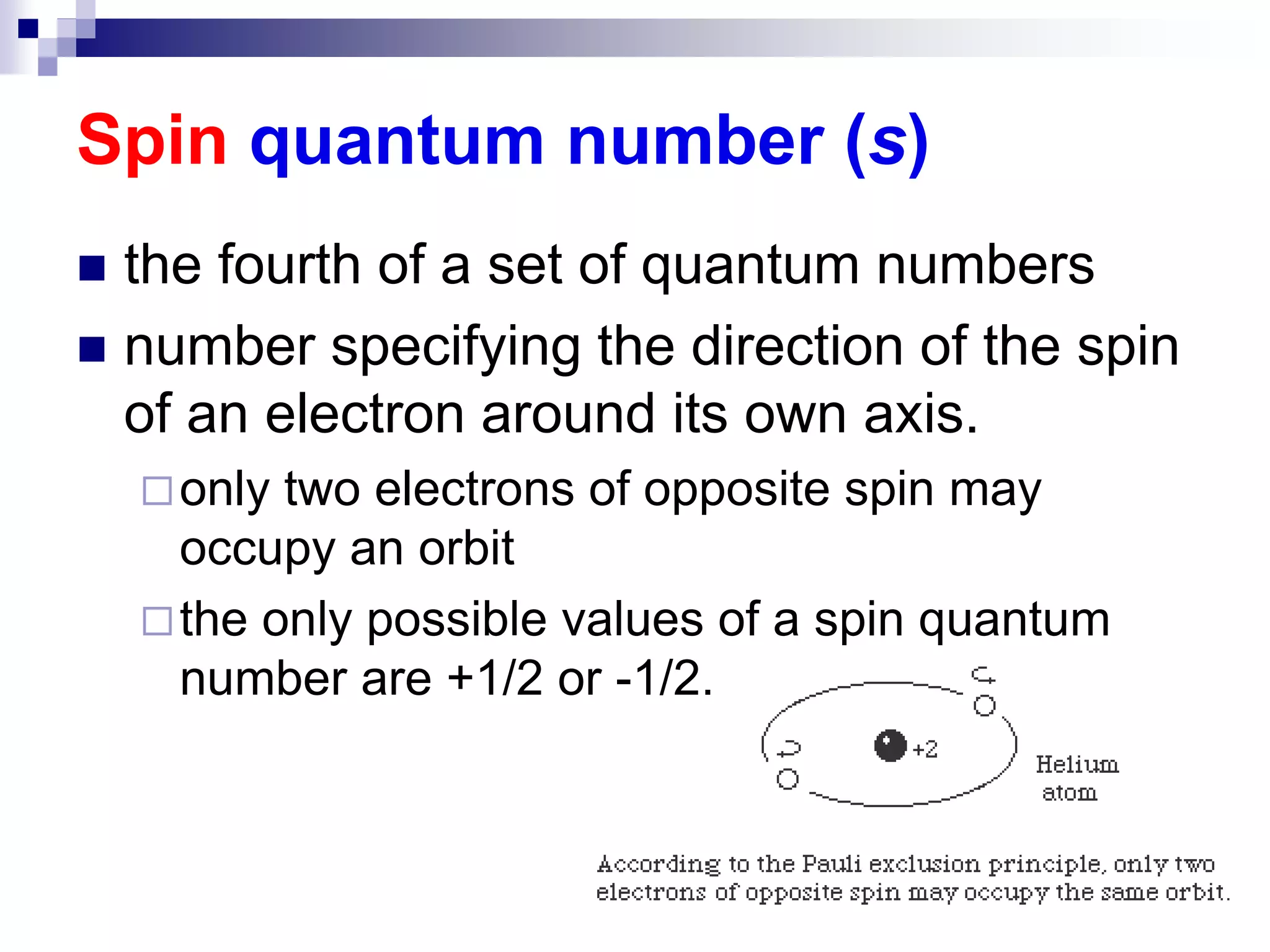
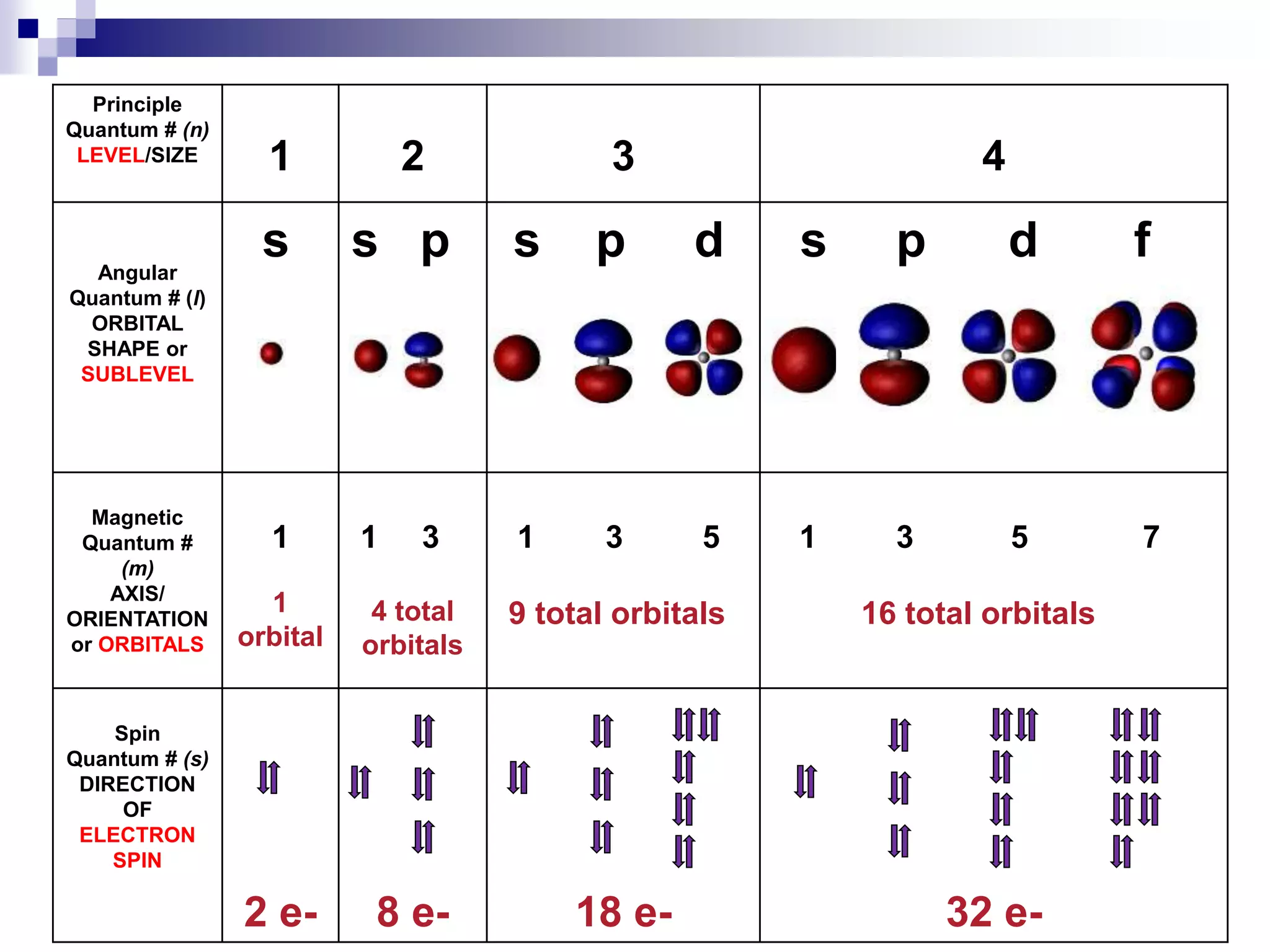
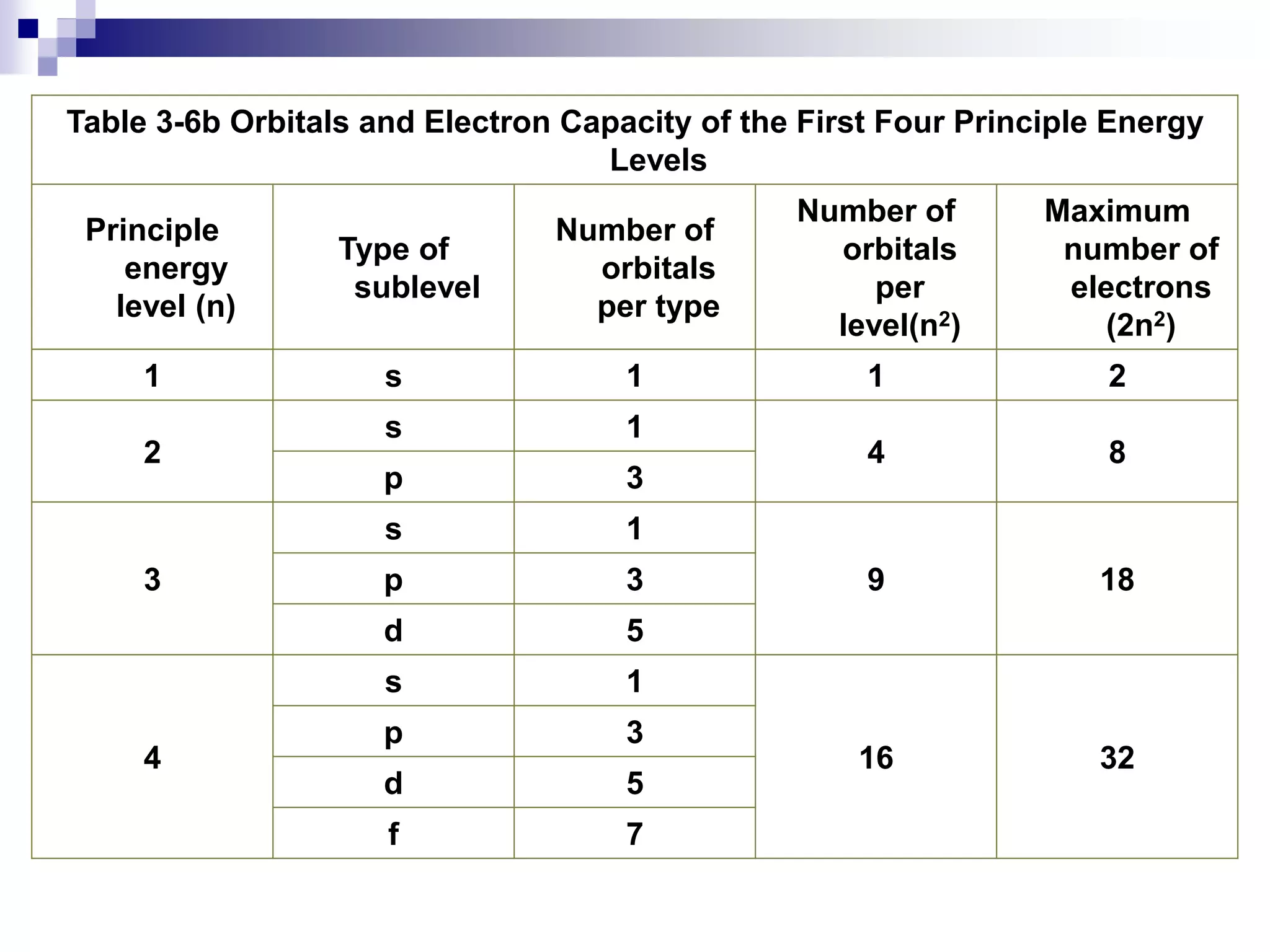
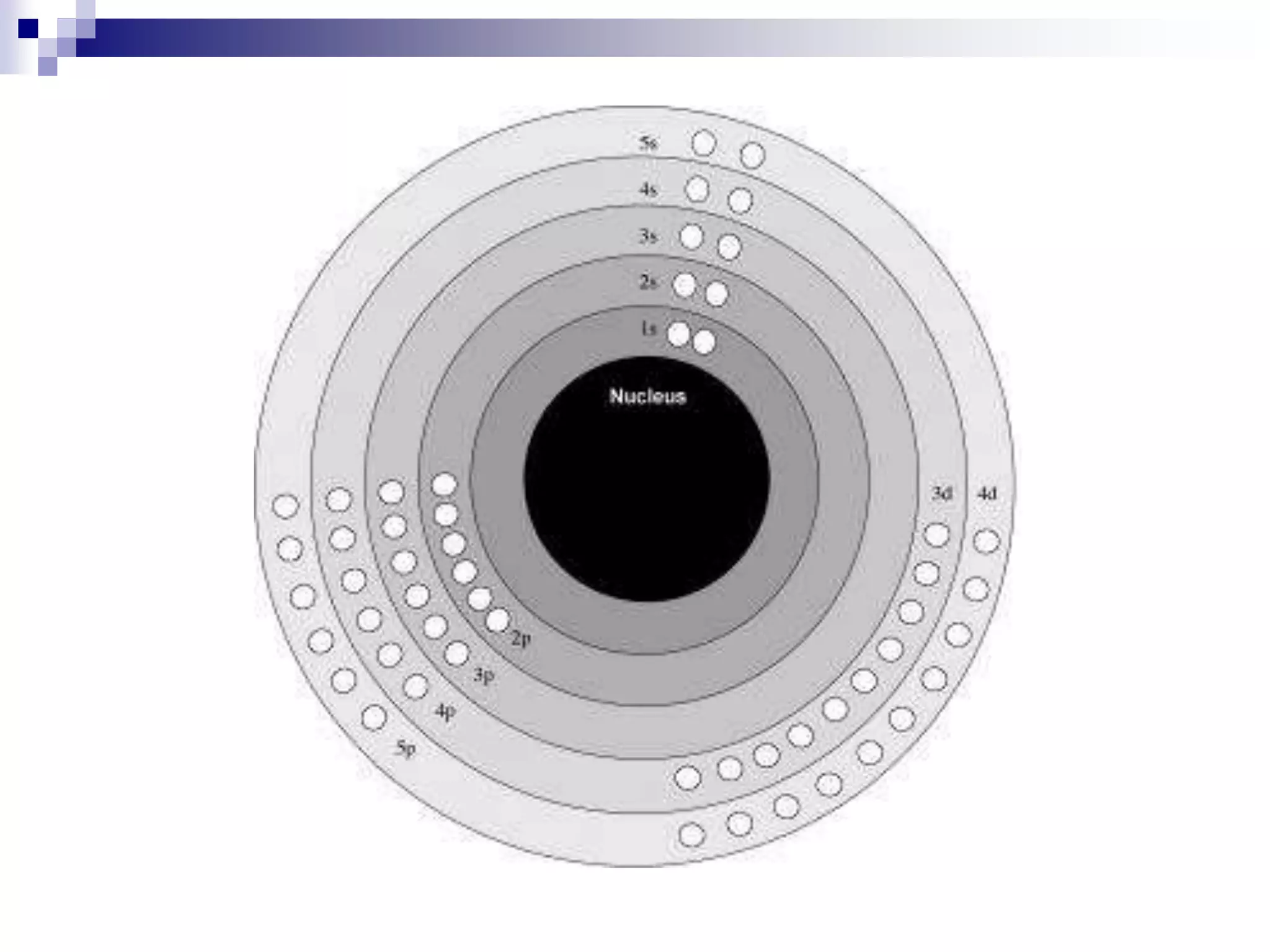
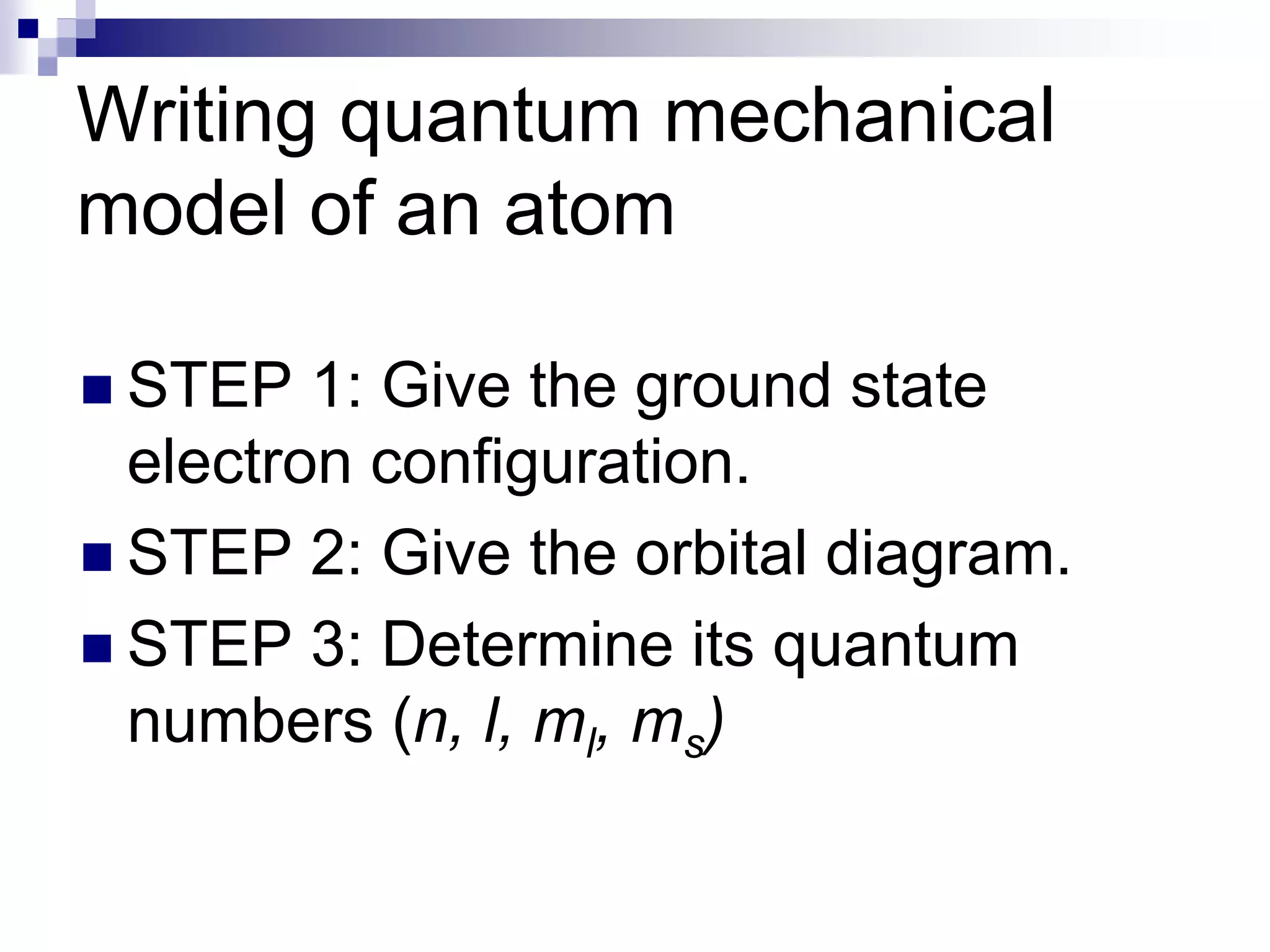
This document provides information on electron configuration and the quantum mechanical model of the atom. It discusses electron configuration notation which uses symbols of orbitals and superscripts to indicate the number of electrons in each orbital. It also covers various principles like the Aufbau principle, Pauli exclusion principle, and Hund's rule. Additionally, it defines the four quantum numbers - principal, angular, magnetic, and spin quantum numbers - which describe the properties of electrons in atomic orbitals. It also gives examples of orbital shapes and capacities for different principle quantum levels.