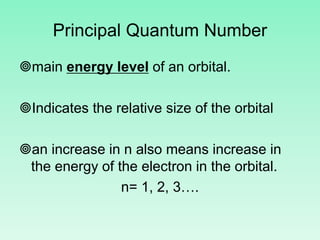
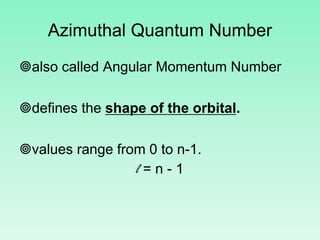
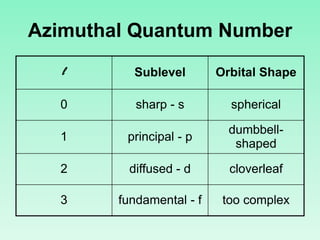
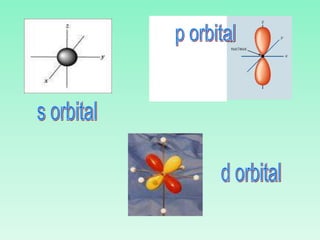
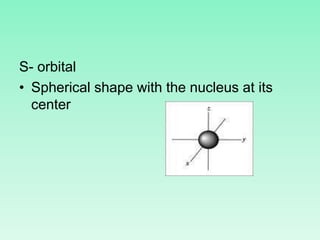
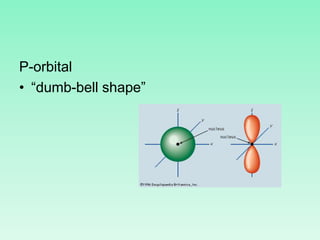
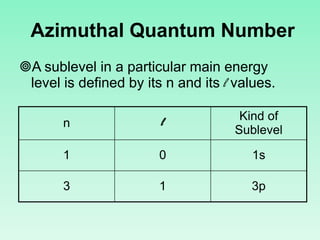
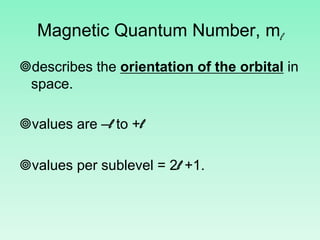
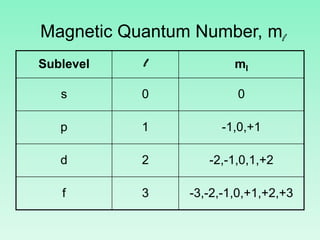
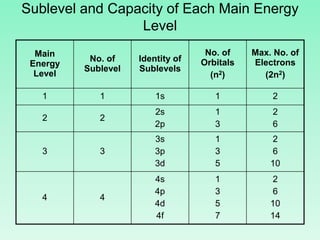
The document discusses quantum numbers which describe the properties of an electron in an atom. There are three main quantum numbers - the principal quantum number n, which indicates the main energy level; the azimuthal quantum number l, which defines the orbital shape; and the magnetic quantum number ml, which describes the orientation of the orbital. Together these quantum numbers uniquely specify each atomic orbital an electron can occupy. The document provides examples of the quantum numbers for different atomic orbitals and energy levels.