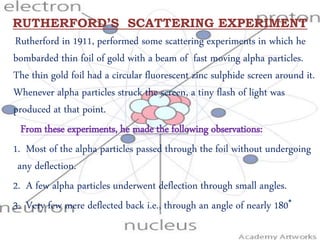
Rutherford performed an experiment in 1911 where he bombarded a thin gold foil with alpha particles. He observed that:
1) Most alpha particles passed through the foil without deflection.
2) A few particles were deflected by small angles.
3) Very few were deflected back at 180 degrees.
From these observations, Rutherford concluded that:
1) Atoms are mostly empty space.
2) A small, dense nucleus explains the few particles being deflected.
3) The nucleus is much smaller than the atom.