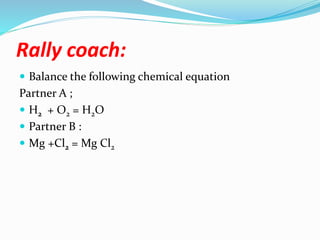
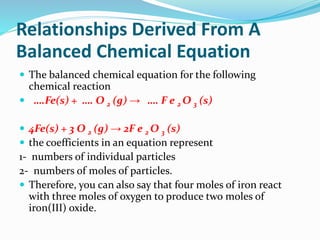
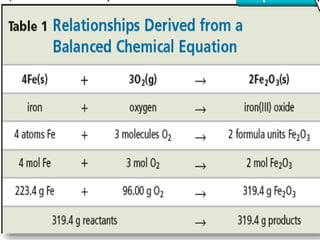
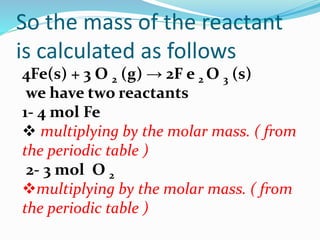
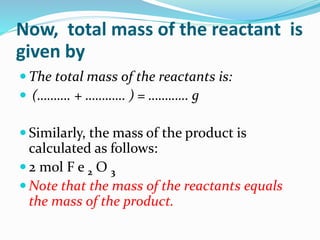
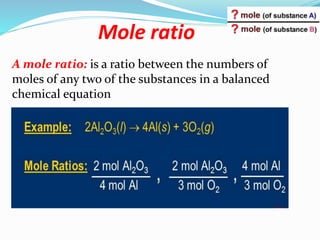
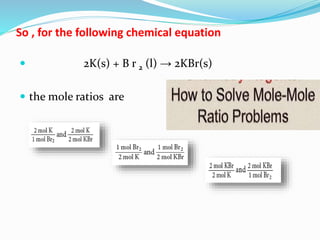
The document discusses relationships derived from a balanced chemical equation. It states that coefficients in a balanced equation represent the numbers of individual particles and moles of particles, indicating mole ratios between reactants and products. Stoichiometry relies on the law of conservation of mass, where the mass of reactants equals the mass of products. Mole quantities can be converted to mass by multiplying by the molar mass to determine the mass relationships between substances in a chemical reaction.