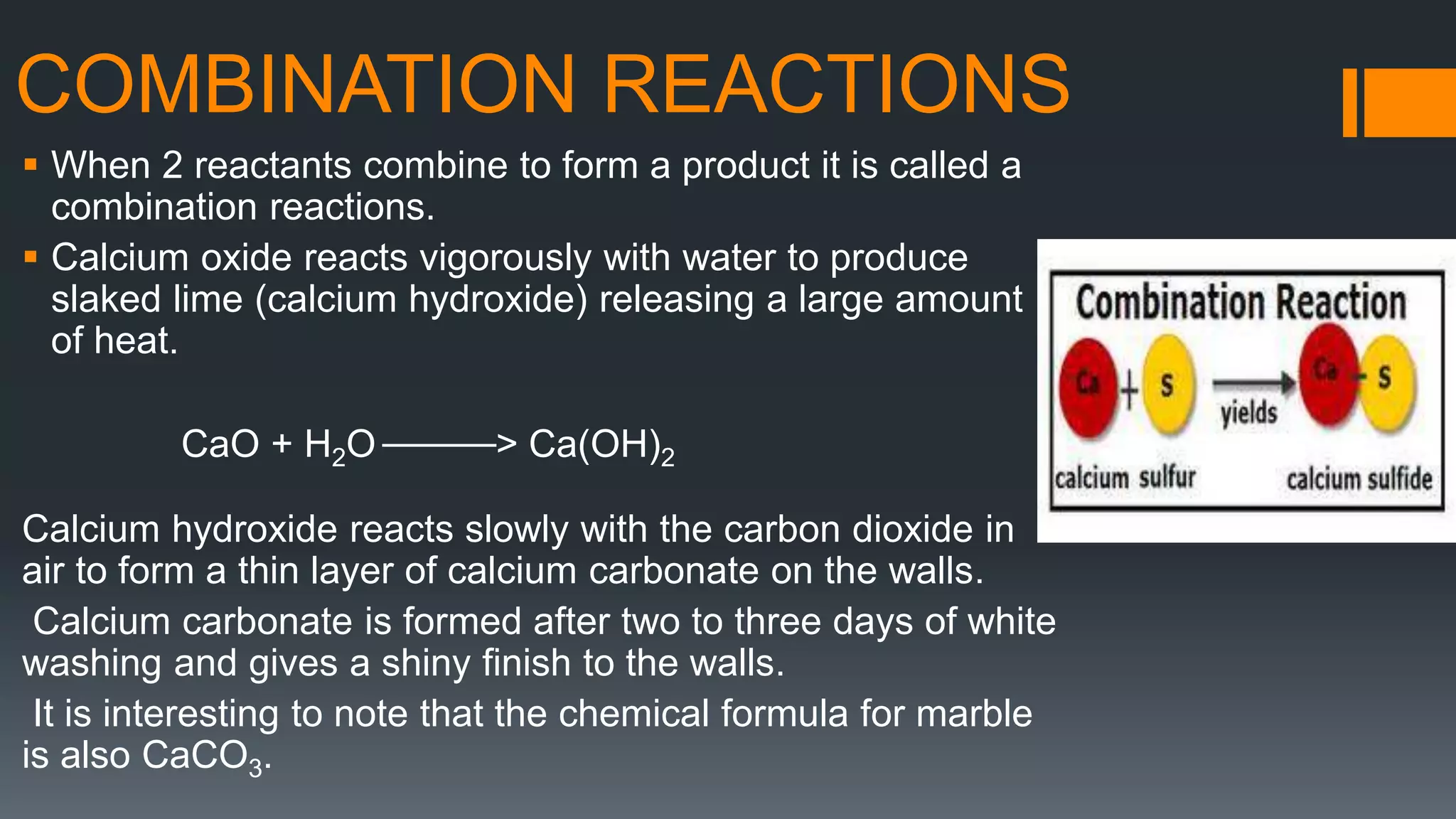
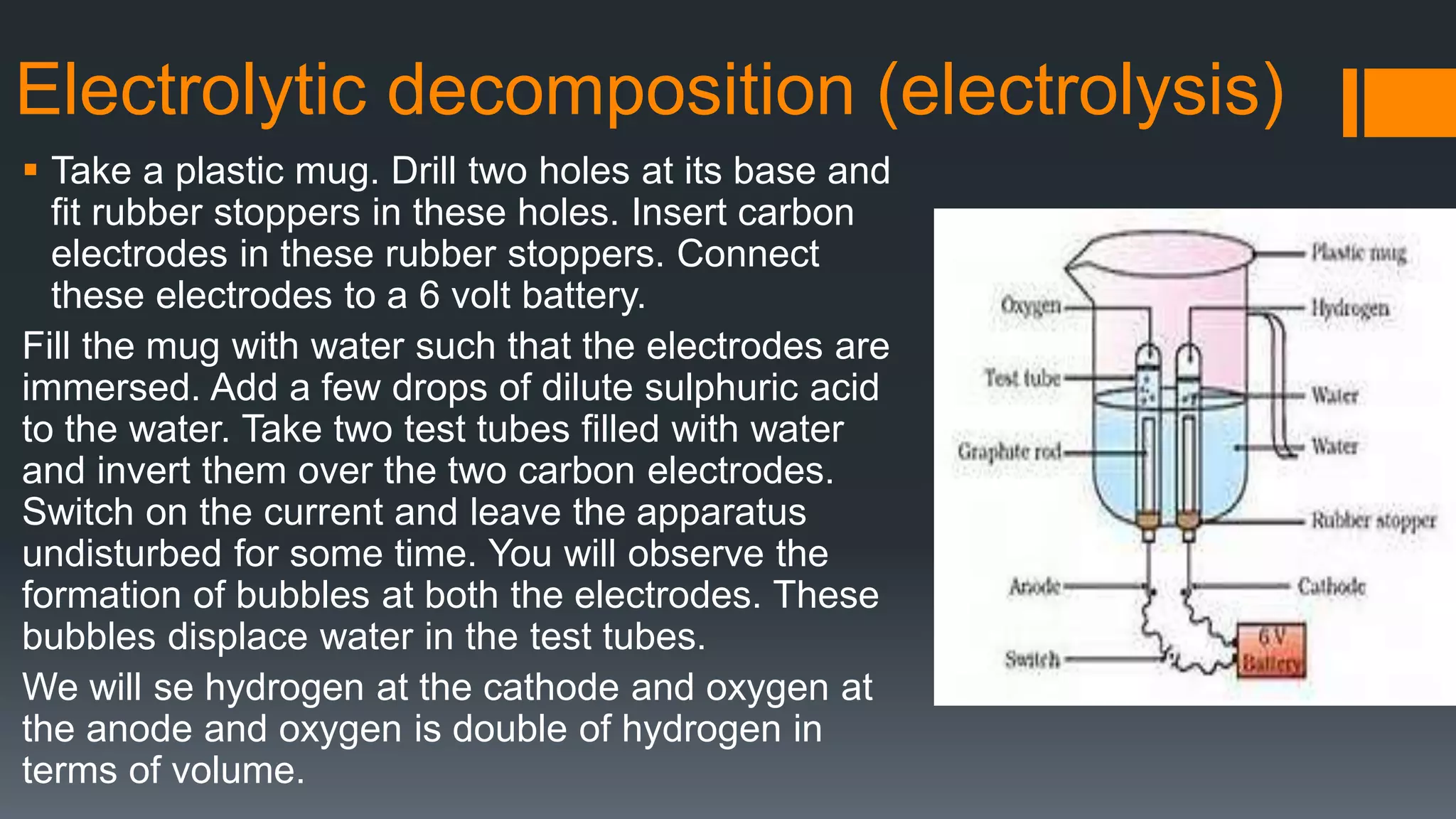
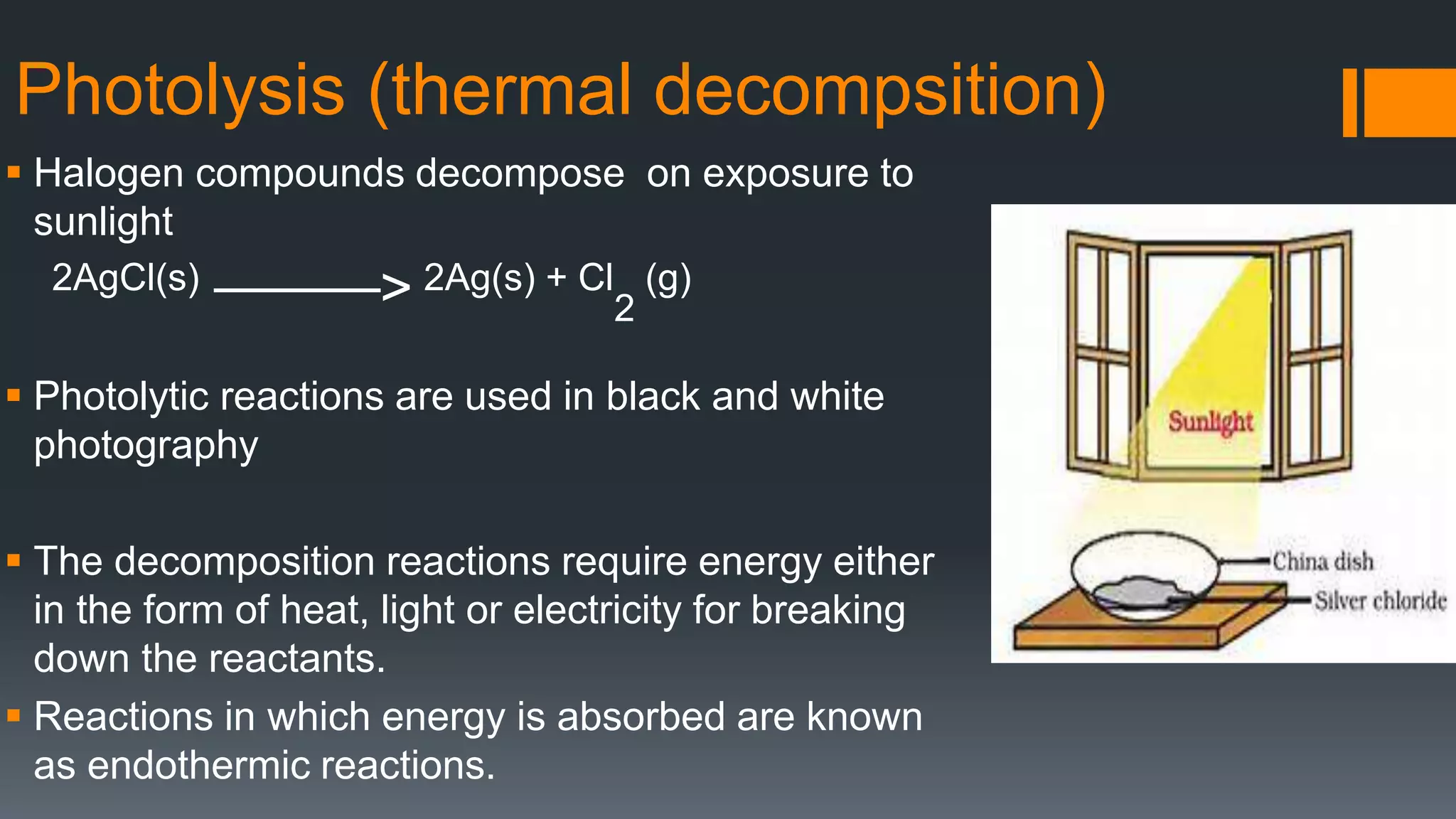
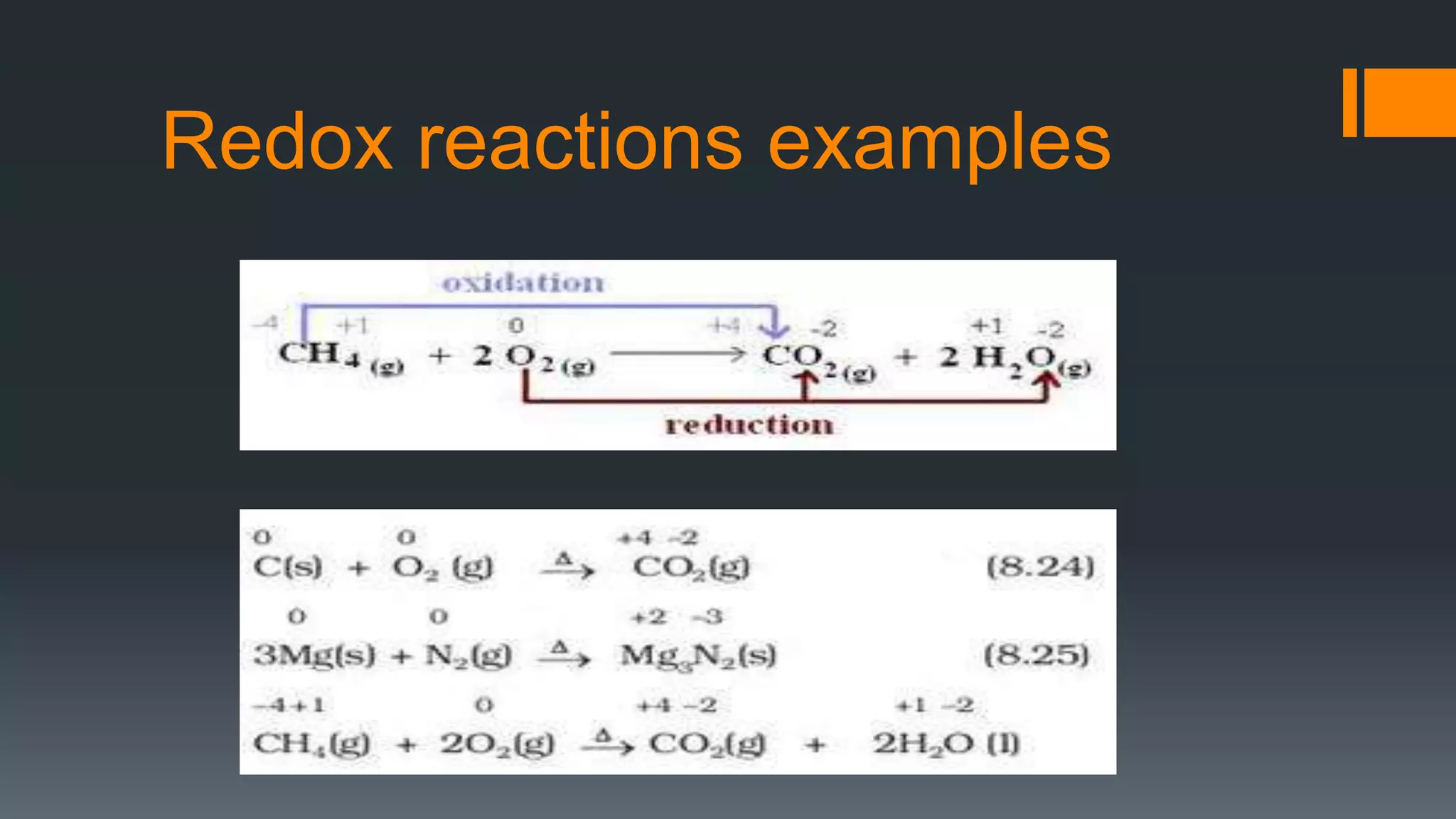
This document discusses chemical reactions and equations. It defines a chemical reaction as a process where reactants are converted into products, which may occur under the influence of a catalyst. It describes how to write chemical equations and balance them by ensuring equal numbers of each type of atom on both sides of the reaction. It also defines and provides examples of different types of chemical reactions: combination, decomposition, displacement, double displacement, and redox reactions. Corrosion and rancidity are discussed as examples of oxidation reactions.