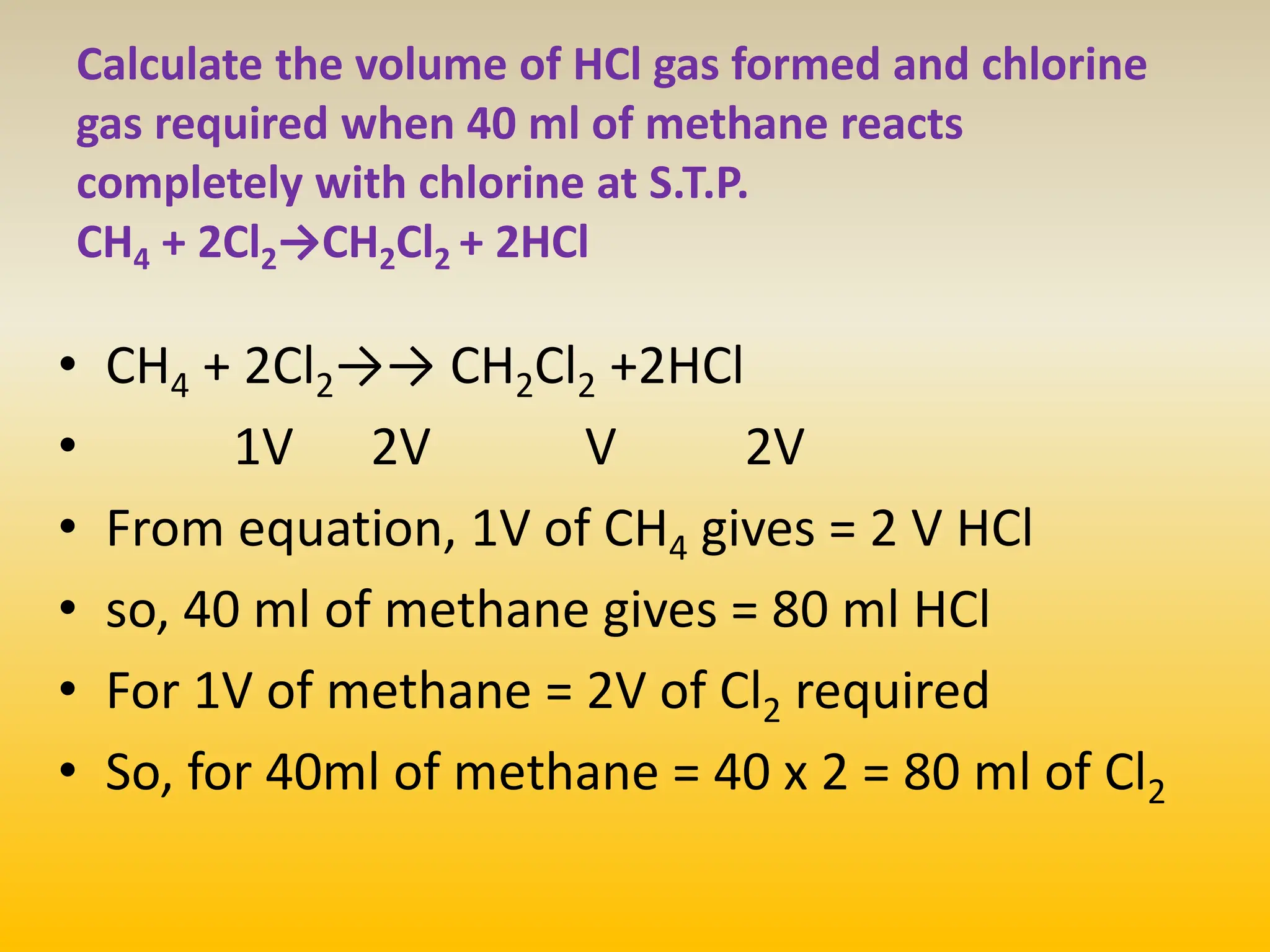
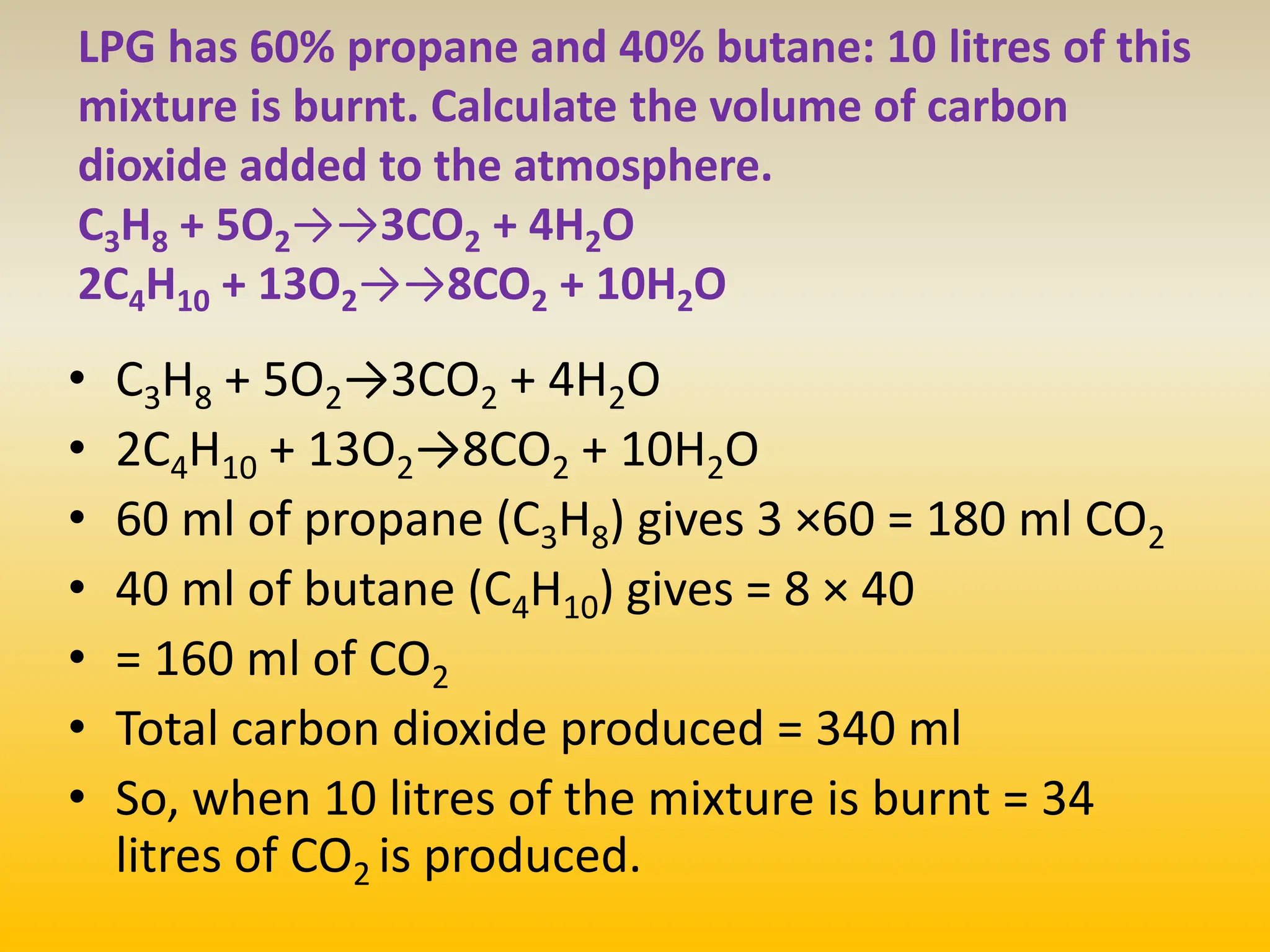
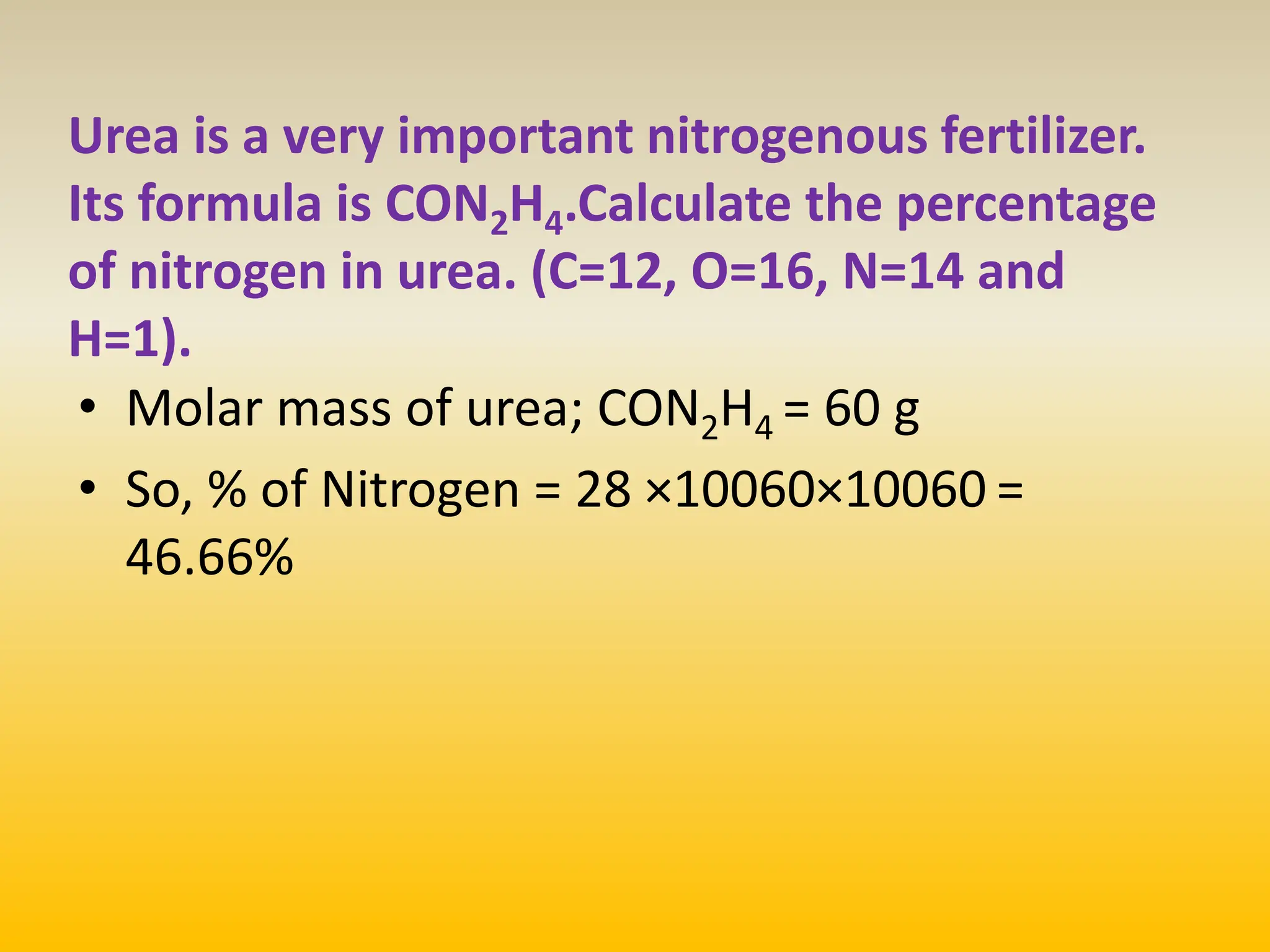
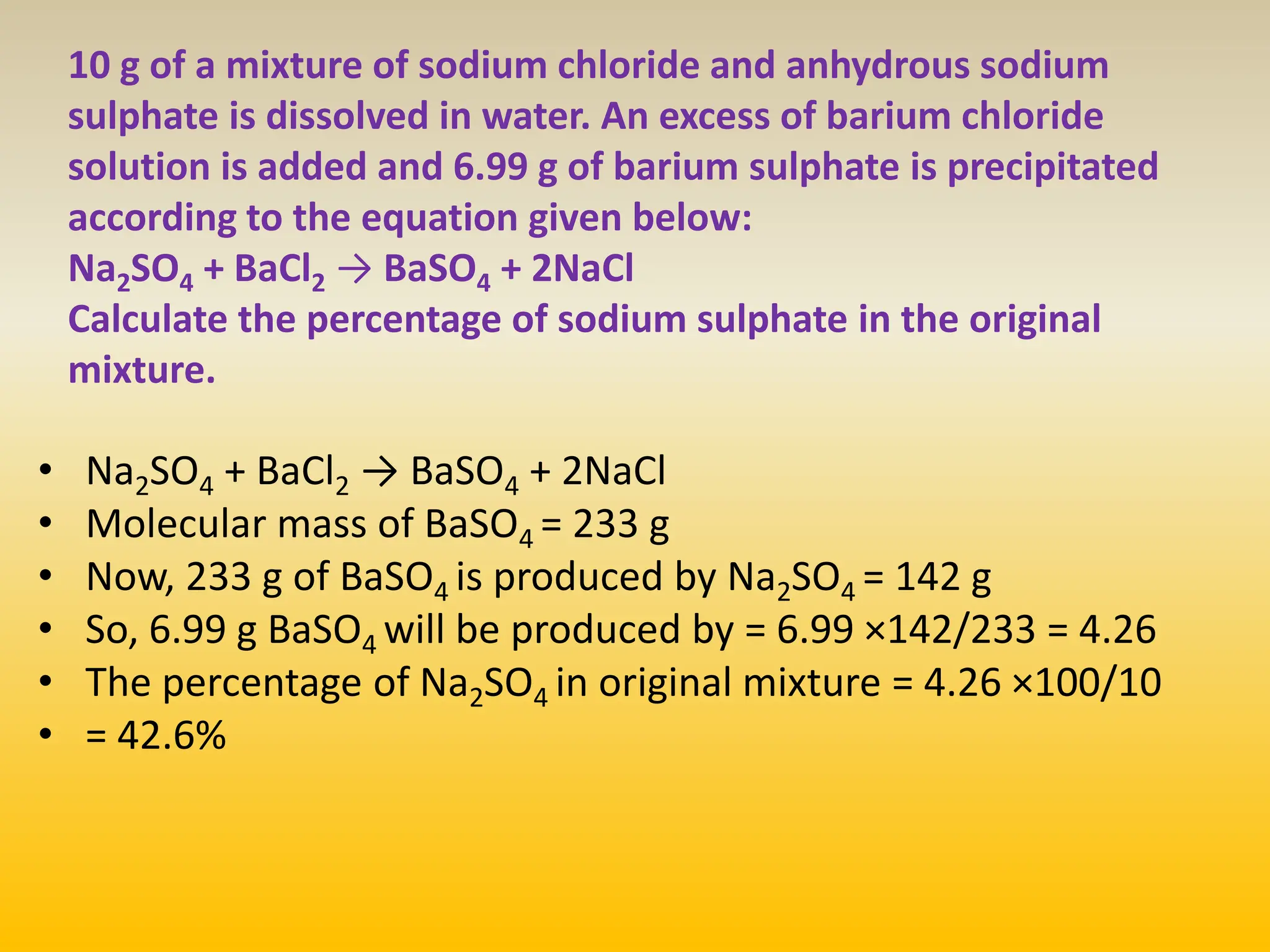
The document contains calculations of volumes and percentages for various chemical reactions at standard temperature and pressure. It summarizes the key steps and results of calculations for reactions involving carbon monoxide, hydrogen, oxygen, ethane, methane, chlorine, nitrogen monoxide, propane, butane, acetylene, urea, hydrogen sulphide, sodium chloride, and sodium sulphate.

![Calculate the volume of oxygen at STP required
for the complete combustion of 100 litres of
carbon monoxide at the same temperature and
pressure.
2CO + O2 ⟶ 2CO2
• From equation:
• 2CO+O2⟶2CO2
• 2 vol.:1 vol.⟶2 vol..
• [By Gay Lussac's law]
• 2 V of CO requires = 1V of O2
• ∴ 100 litres of CO requires = 1/2 x 100 = 50 litres.
• Hence, required volume of oxygen is 50 litres.](https://image.slidesharecdn.com/presentation1-240222165502-ae7c0060/75/Presentation1-numerical-problems-pptx-2-2048.jpg)
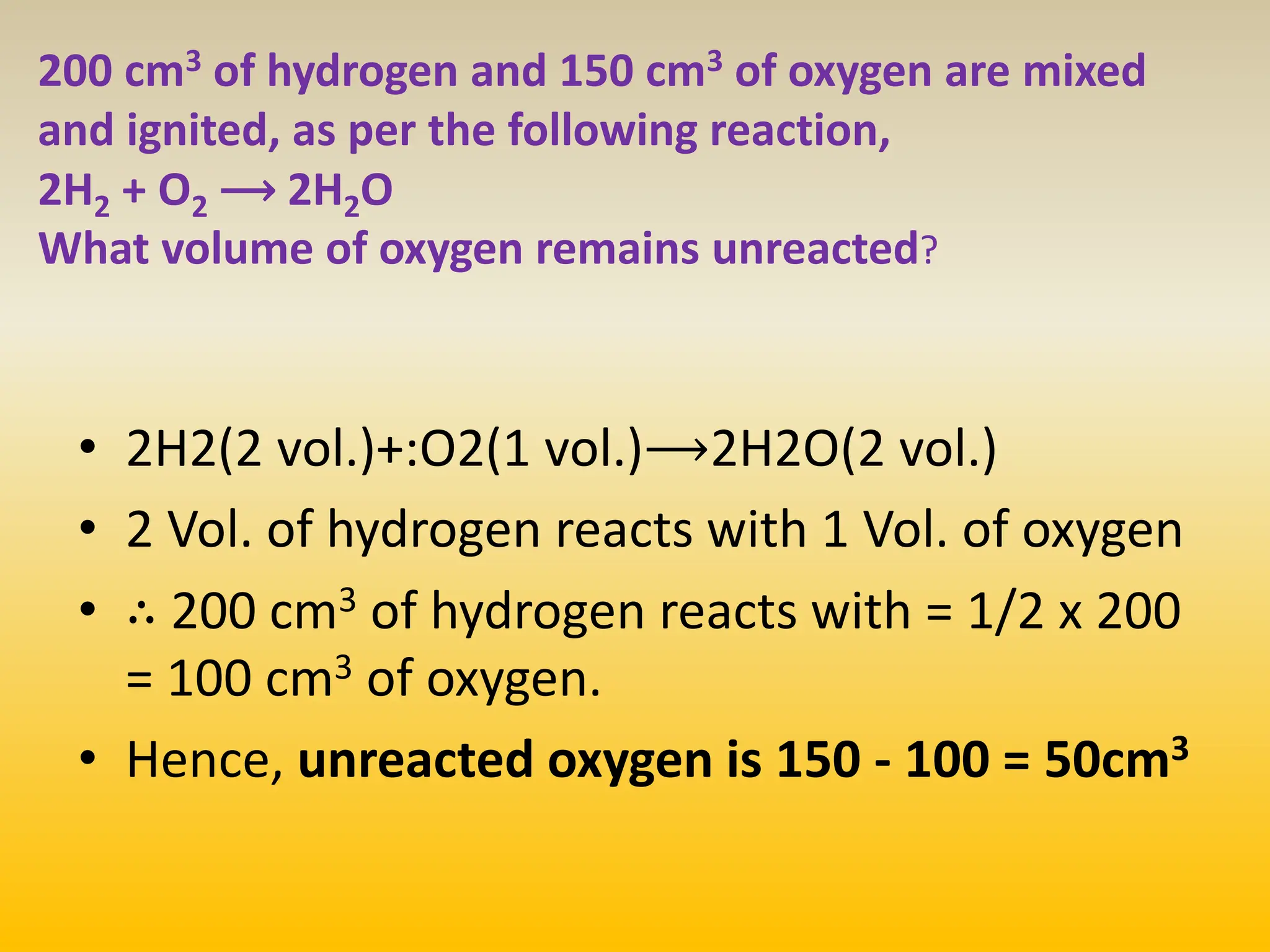
![1250 cc of oxygen was burnt with 300 cc of ethane
[C2H6]. Calculate the volume of unused oxygen and the
volume of carbon dioxide formed:
2C2H6 + 7O2 ⟶ 4CO2 + 6H2O
• 2C2H6(2 vol.)+:7O2(7 vol.)⟶4CO2(4 vol.)+6H2O
• [By Gay Lussac's law]
• 2 Vol. of C2H6 requires 7 Vol. of oxygen
• ∴ 300 cc C2H6 will require= 7/2 x 300
• = 1050 cc of Oxygen
• Hence, unused oxygen = 1250 - 1050 = 200 cc
• Similarly,
• 2 Vol. of C2H6 produces 4 Vol. of carbon dioxide
• ∴ 300 cc C2H6 produces =4/2x300
• = 600 cc of Carbon dioxide
• Hence, carbon dioxide produced = 600 cc.](https://image.slidesharecdn.com/presentation1-240222165502-ae7c0060/75/Presentation1-numerical-problems-pptx-4-2048.jpg)