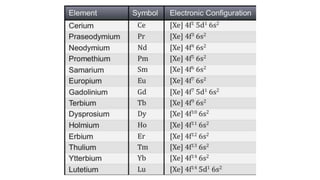
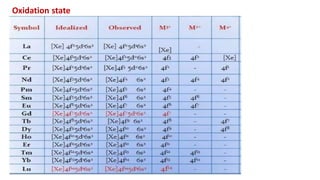
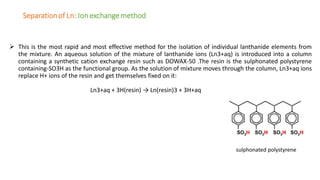
The document discusses lanthanide contraction, which occurs due to the ineffective shielding of outer electrons by the 4f electrons as atomic number increases, leading to a decrease in ionic size. It highlights the challenges in separating lanthanides from one another due to their similar size and charge, outlining methods for separation such as ion exchange using synthetic resins and elution with complexing agents. The document emphasizes that smaller lanthanide ions are more firmly bound to the resin, affecting their separation efficiency.