This document describes the Kjeldahl method for determining nitrogen content. It involves three main steps: 1) Digestion breaks down organic compounds to liberate nitrogen as ammonium sulfate. 2) Distillation converts ammonium ions to gaseous ammonia. 3) Titration determines the total nitrogen amount by titrating with a standard sodium hydroxide solution. The method is demonstrated through a sample problem calculating the percentage of urea in a sample based on its nitrogen content.


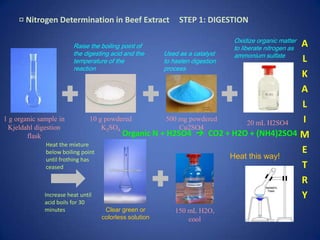




![¤ Nitrogen Determination in Beef Extract
A
L
K
A
L
I
M
E
T
R
Y
Sample Problem:
A 0.200g of a urea (FW= 60) sample is analyzed by the Kjeldahl method. The
ammonia collected in a 50 mL of 0.05 M H2SO4 . The excess acid required 3.4 mL
of 0.05 M NaOH. Find the percentage of the compound in the sample.
Solution:
½ mmol NH3 = (0.05 M × 50mL) ˗ ½(0.05 M 3.4mL)
mmol NH3 = 2[(0.05 M × 50mL) ˗ ½(0.05 M 3.4mL)]
mmol urea = ½ mmol NH3
mmol urea = (0.05 M × 50mL) ˗ ½(0.05 M 3.4mL) = 2.415 mmol](https://image.slidesharecdn.com/kjeldahlmethod-130928132954-phpapp02/85/Example-Kjeldahl-Method-8-320.jpg)

