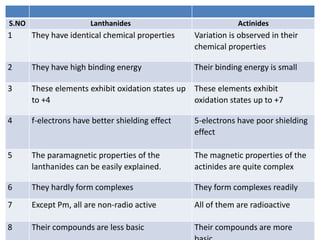
The document discusses inner transition elements, specifically the lanthanide and actinide series. It provides details on their electronic configurations, oxidation states, properties such as color and magnetism, extraction from monazite sand, and separation methods. It also compares the lanthanides and actinides, noting they both show lanthanide/actinide contractions and have similar properties, but the actinides exhibit more variable chemistry and are all radioactive.