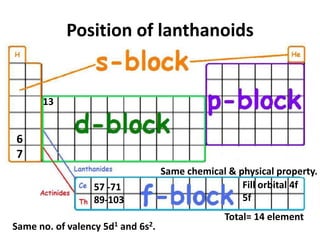
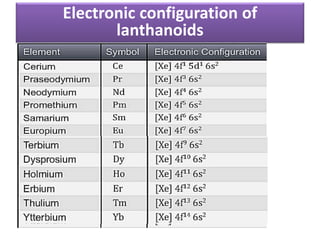
The document discusses the lanthanide series of f-block elements. It provides the electronic configurations of the lanthanide elements from Lanthanum to Lutetium. It describes the lanthanide contraction effect where atomic and ionic radii decrease across the series. Key effects of lanthanide contraction include decreased basicity and similar ionic radii of post-lanthanide elements to those in the previous period. The document also briefly introduces the actinide series and notes their similar properties to lanthanides but with 5f electrons instead of 4f.










![Electronic configuration of
lanthanoids
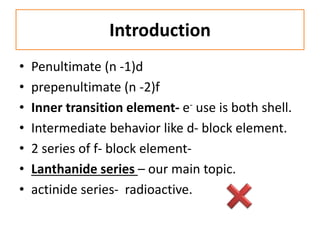
• E.C Based on aufbau principle .
La (57)
1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 5d1 6s2
[Xe] – 54
1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6
The electronic configuration of Ln is
[Xe] 4f0 5d1 6s2 or [Xe] 4f0
Similarly Ce- 4f1, Pr- 4f2,……… Lu-4f14.(expected)
but observed E.C is not true for all element.](https://image.slidesharecdn.com/xxevw3fyszchp8cb2hck-signature-016162306cab773303228479b063befdf29baf20ea52f9a91b002e88ad5a07bd-poli-151129105602-lva1-app6891/85/F-block-element-14-320.jpg)
![Oxidation state
• Common oxidation state is +3 more stable.
• Loses of two 6s electron and one 5d or 4f
electron.
• Some either show +2 or +4 Oxidation state.
• Ln [Xe] 4f0 5d1 6s2 and Ln+3 [Xe]4f0 5d0 6s0
Ln, Pm, Ho, Eb, Lu +3
Ce, Pr, Tb, Dy +3, +4
Sm, Eu, Tm, Yb +2, +3
Nd, +2, +3, +3](https://image.slidesharecdn.com/xxevw3fyszchp8cb2hck-signature-016162306cab773303228479b063befdf29baf20ea52f9a91b002e88ad5a07bd-poli-151129105602-lva1-app6891/85/F-block-element-16-320.jpg)













![Electronic configuration
• Ac (89)-
1s22s22p63s23p63d104s24p64d105s25p64f145d106s26
p66d17s2.
• [Rn]- 86
• 1s22s22p63s23p63d104s24p64d105s25p64f145d106s26
p6
• Ac electronic configuration is
• [Rn] 6d17s2 or [Rn] 5f0 6d1 7s2
• 6d and 7s electron is same only 5f electron
change by increasing order. Th(90)- [Rn] 5f1,……..
5f10.](https://image.slidesharecdn.com/xxevw3fyszchp8cb2hck-signature-016162306cab773303228479b063befdf29baf20ea52f9a91b002e88ad5a07bd-poli-151129105602-lva1-app6891/85/F-block-element-30-320.jpg)


