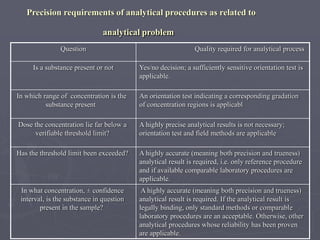
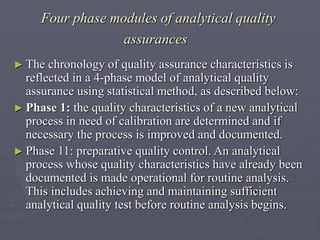
This document discusses quality control of medicinal products. It defines quality control as procedures to ensure identity and purity of pharmaceuticals, ranging from simple chemical tests to complex pharmacopoeial standards. The quality of products can deviate and require analysis to determine specifications. Counterfeit medicines may contain no active ingredient, wrong ingredient, low quantity, or be manufactured poorly. Pharmacopoeial standards and methods provide specifications for identity, purity, assays, and other quality tests for drugs. Quality control is important for pharmaceutical manufacturers, clinical analysis, law enforcement, and ensuring safety and efficacy of medicines.


















![►
This requires the development of valid test methods to
measure relevant quality parameters and the
application of generally accepted criteria to assess the
quality. However, if the laboratory uses different
analytical techniques to those used by the
manufacturer [e.g. a high performance liquid
chromatographic (HPLC) technique instead of an
ultraviolet (UV) spectrophotometric technique], the
results may differ significantly from those obtained by
the manufacturer, particularly if high levels of
impurities are present](https://image.slidesharecdn.com/qualitycontrol-140218132313-phpapp02/85/Quality-control-of-pharmaceuticals-21-320.jpg)










