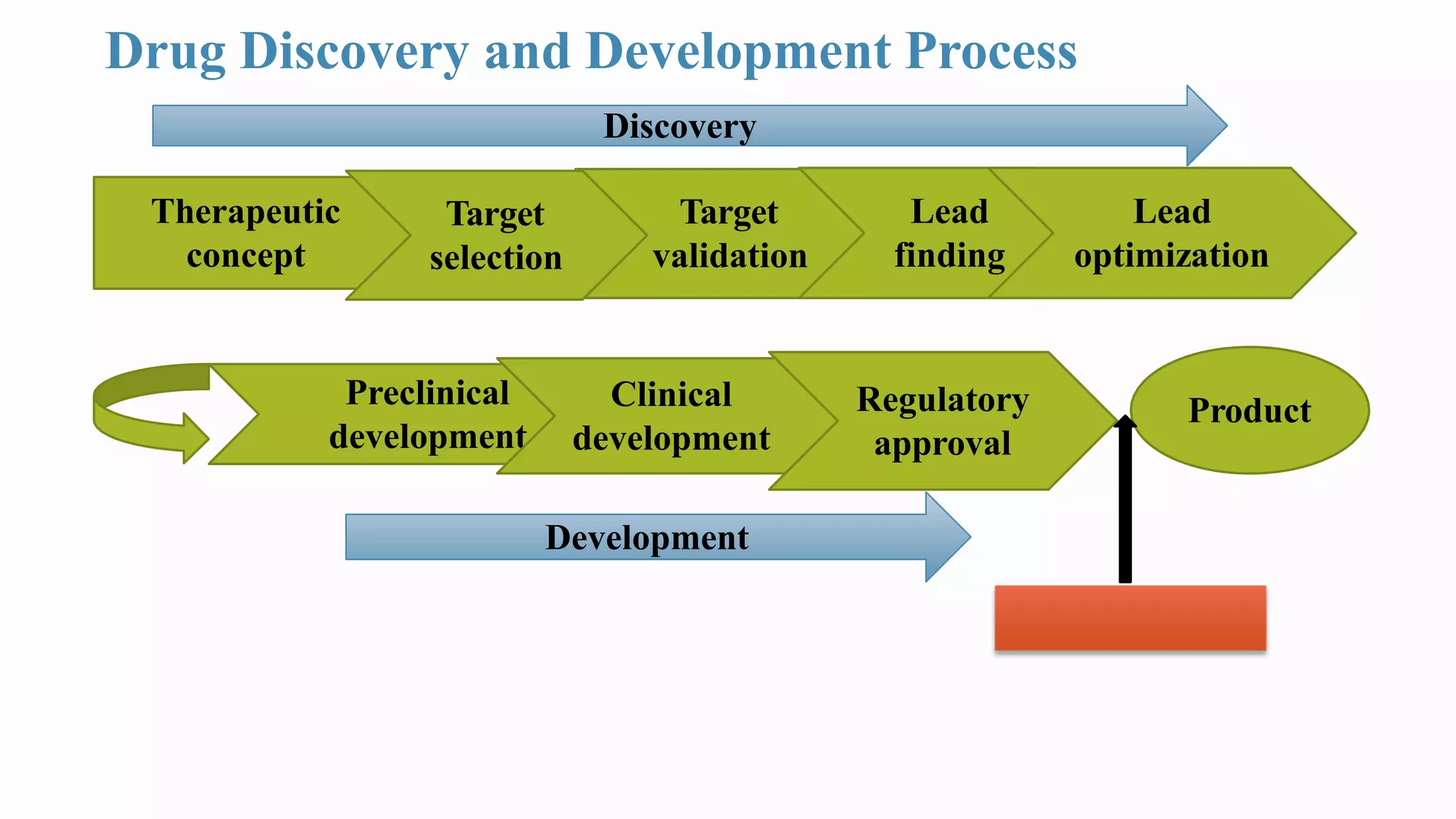
Good Laboratory Practice (GLP) regulations were created by the FDA in the 1970s in response to cases of poor laboratory practices and fraudulent activities in safety testing. GLP aims to ensure that non-clinical safety studies are well-planned, performed, monitored, recorded, and reported in a uniform manner to assure data quality and integrity. Key aspects of GLP include requirements for facilities, equipment, standard operating procedures, personnel training, test system characterization, record keeping, and quality assurance programs. Following GLP standards helps generate reliable and mutually accepted non-clinical data to support regulatory approval of products.