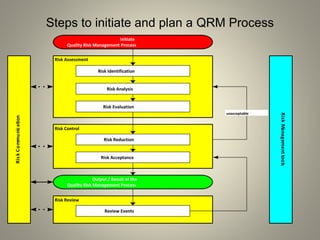
This document provides an overview of quality risk management. It defines key terms like risk, hazard, risk assessment, and risk management. It describes the ICH guidelines related to quality risk management and explains the scope, principles, flow chart, and tools of quality risk management. The tools discussed include FMEA, FMECA, FTA, HACCP, and basic risk management methods. The document aims to introduce the topic of quality risk management.