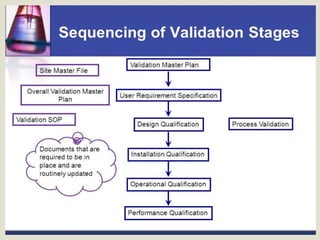
The document discusses developing a validation master plan (VMP) for a new pharmaceutical facility. Key points:
- A VMP comprehensively describes validation requirements and plans for meeting them. It covers production, storage, utilities, and staff areas.
- The VMP sets goals and limits for validation projects. It defines the scope and systems included.
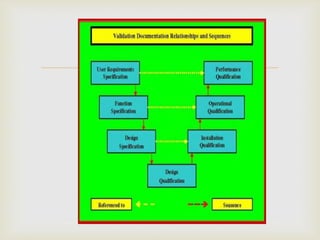
- Developing the VMP involves determining standards, qualifications for design, installation, operation, and performance, documentation requirements, and change control procedures.
- User requirement specifications (URS) are critical documents that validation is dependent on. Developing clear, testable URS in multiple levels is important, especially for software.











![
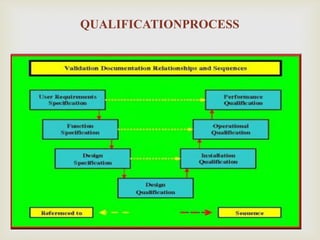
URS [User Requirement Spécifications]
DQ [Design Qualification]
IQ [Installation Qualification]
OQ[Operation Qualification]
PQ [Performance Qualification]
BASIC STEPS OF
QUALIFICTION](https://image.slidesharecdn.com/validationmasterplan-191110071655/85/Validation-master-plan-14-320.jpg)
![
Once the end user requirements specification or URS as it
is commonly called; is documented, agreed and approved
they form the basic URS Level-1 document. The engineers
(or vendor) can then commence the preliminary design to
establish exactly what functions are required for each of
the items specified in the user requirements specification,
the end user has listed. Once this functionality is
documented and approved it forms the URS Level-2
document. This is the final level of the URS unless
software is used.
USER REQUIREMENT
SPECIFICATION [URS]](https://image.slidesharecdn.com/validationmasterplan-191110071655/85/Validation-master-plan-16-320.jpg)
![
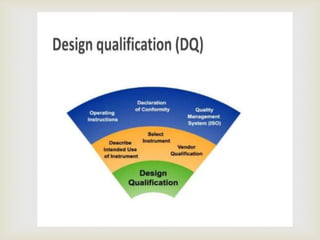
Design Qualification is used at the stage where a design
that has been developed from the URS, is reviewed and
documented by competent persons to ensure that the
designed equipment, if built, will satisfy all the detailed
specified requirements. The Design Qualification is the
only document that is going to confirm that the design will
work. It must be carried out by qualified people who can
challenge the design performance. If you have no such
persons on your staff you must contract them in, or
contract the DQ out
DESIGN
QUALIFICATION[DQ]](https://image.slidesharecdn.com/validationmasterplan-191110071655/85/Validation-master-plan-17-320.jpg)
![
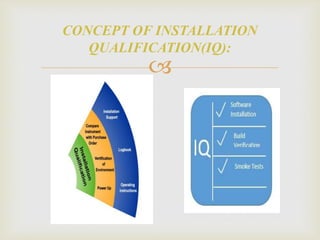
The Installation Qualification (IQ) execution; verifies that the
equipment, and its ancillary systems or sub-systems have been
installed in accordance with installation drawings and or
specifications. It further details a list of all the
CGMPrequirements that are applicable to this particular
installation qualification. These requirements must all be
satisfied before the IQ can be completed and the qualification
process is allowed to progress to the execution of the
Operational Qualification (OQ).
INSTALLATION QUALIFICATION [IQ]](https://image.slidesharecdn.com/validationmasterplan-191110071655/85/Validation-master-plan-18-320.jpg)
![
This Operational Qualification (OQ)protocol comes with an
interactive SOP as a prefixed document. In the preparation of
the Operational Qualification validation protocols, it is
important to allow a degree of flexibility. Should the OQ remain
untouchable until the Installation-Qualification (IQ) is
completed and signed off. The Operational Qualification
includes a review of the Standard Operating Procedure (SOP's)
for startup, operation, maintenance, safety, and cleaning /
sanitization as applicable, must they be in fully approved
format? These flexibilities must be built into the qualification
process.
OPERATIONQUALIFICATION[OQ]](https://image.slidesharecdn.com/validationmasterplan-191110071655/85/Validation-master-plan-19-320.jpg)
![
The normal expectations for PQ are given as requiring,
documented verification that facilities, systems and
equipment, as connected together, can perform effectively
and reproducibly, based on the approved process method
and product specification. Onto that now should be grafted
The verification that the all the requirements specified in
theUser Requirements Specification (URS) have been
fully complied
PERFORMANCE QUALIFICATION[PQ]](https://image.slidesharecdn.com/validationmasterplan-191110071655/85/Validation-master-plan-20-320.jpg)