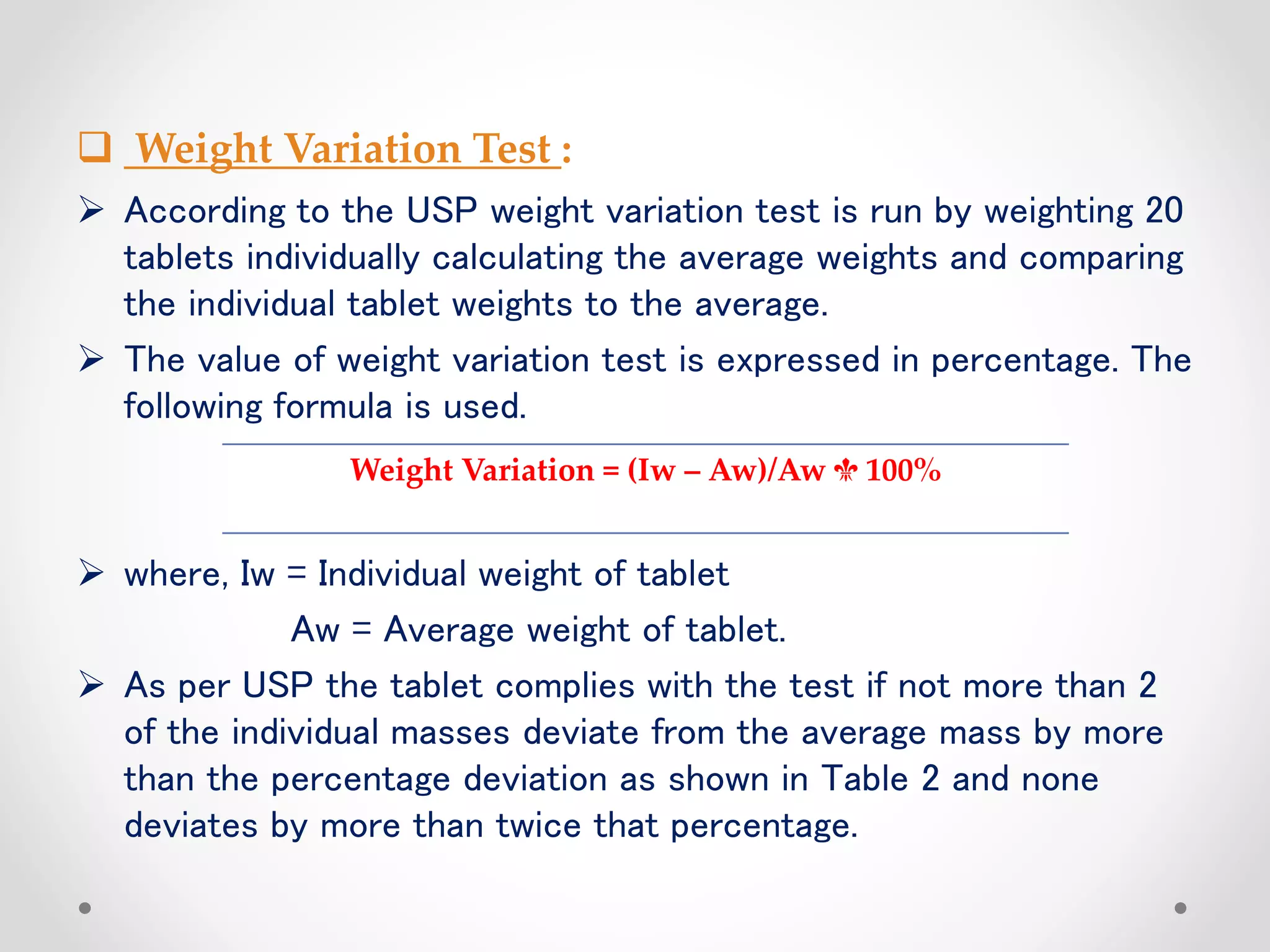
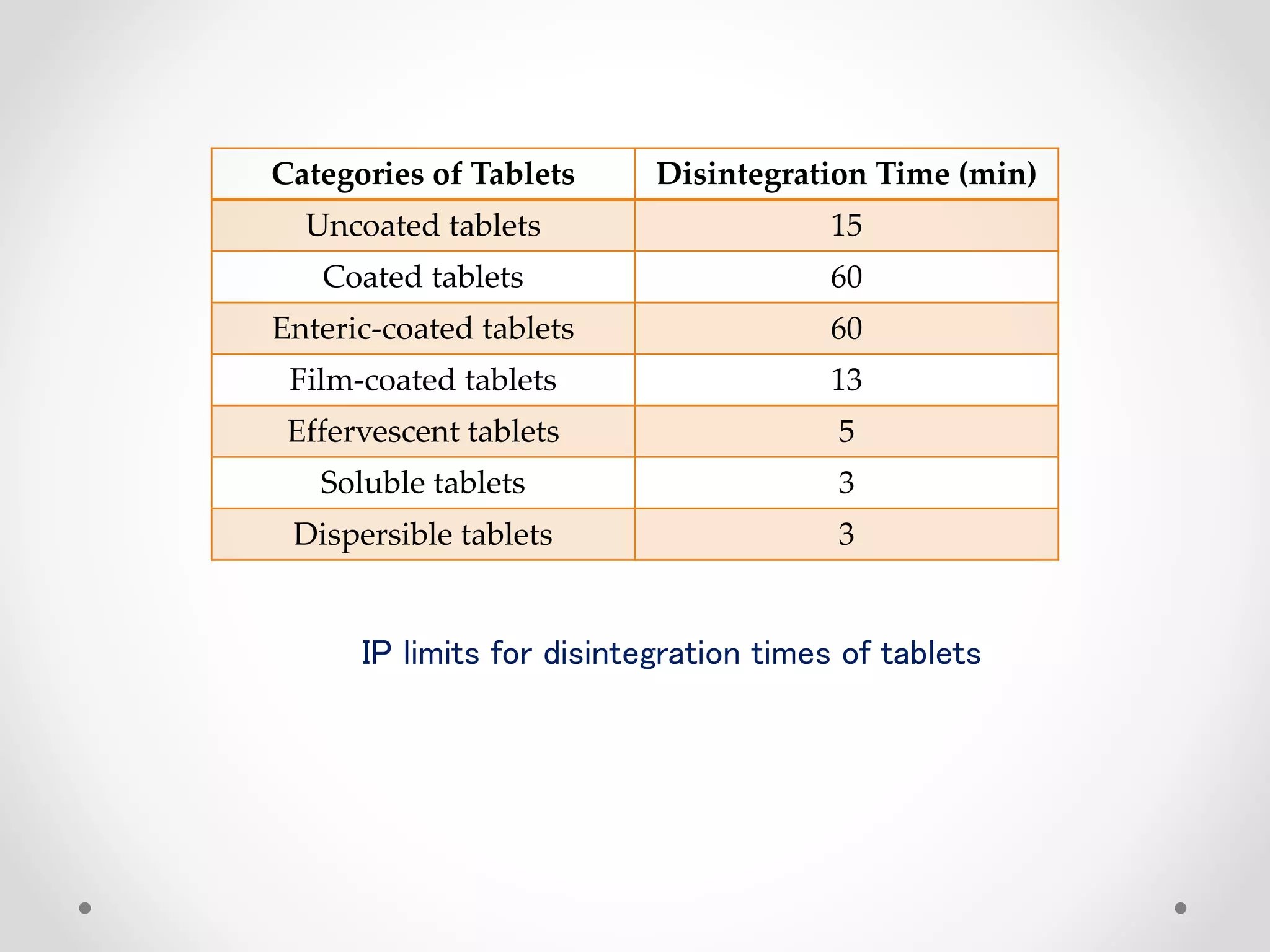
This document discusses in-process quality control (IPQC) and finished product quality control (FPQC) for pharmaceutical products. It defines IPQC and outlines its objectives to optimize processes, monitor operations, and inspect raw materials, equipment, environment and more. Key IPQC tests are described including physical/chemical tests (identity, quality, purity, potency), biological/microbiological tests. Specific IPQC parameters and checks are provided for tablets, including tests for size/shape, color, thickness, assay, dissolution and more. Acceptance criteria are defined according to pharmacopeial standards. The importance of IPQC for ensuring quality products is emphasized.