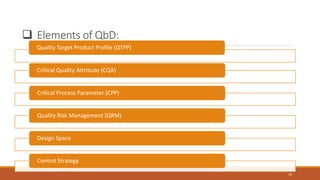
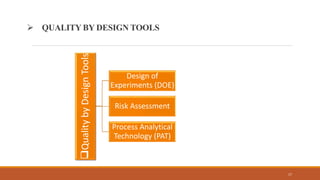
The document discusses Quality by Design (QbD) in pharmaceutical development, emphasizing its systematic approach that integrates predefined objectives and risk management to enhance product and process understanding. It outlines objectives, advantages, key elements like Quality Target Product Profile (QTPP) and Critical Quality Attributes (CQA), and QbD tools such as Design of Experiments (DOE) and Process Analytical Technology (PAT). The conclusion highlights QbD's growing significance in ensuring quality medicines while improving manufacturing efficiency and regulatory compliance.