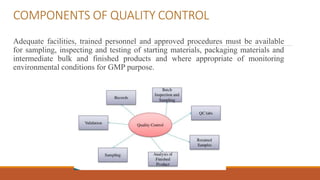
The document discusses quality management, assurance, and control within the pharmaceutical industry, emphasizing the importance of maintaining high quality and safety standards in drug production. It outlines key components of quality control, including the establishment of quality standards, evaluation of production processes, and adherence to good manufacturing practices (GMP). Additionally, it details total quality management (TQM) principles that involve the participation of all employees to enhance organizational performance and customer satisfaction.