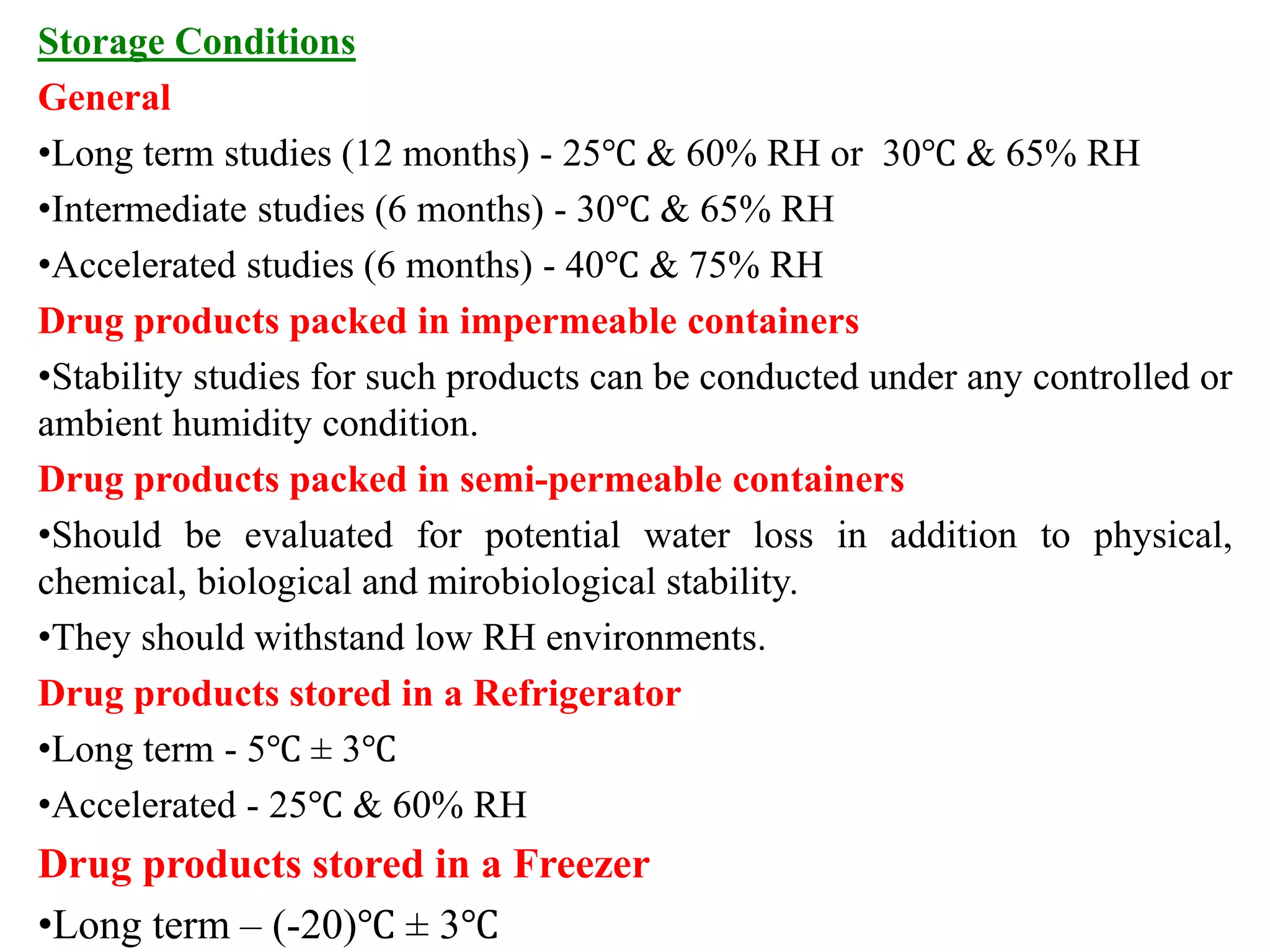
The document provides an introduction to ICH guidelines and stability testing guidelines. It discusses that ICH aims to harmonize technical requirements for pharmaceuticals across regions to eliminate unnecessary delays and ensure safety. ICH has six participants representing regulatory bodies and industry. Guidelines are developed on quality, safety, efficacy and other topics. Quality guidelines (Q series) focus on chemical and pharmaceutical quality assurance. Stability testing guidelines provide guidance on conducting stability studies to ensure quality, safety and efficacy of drug products over time under various environmental conditions.