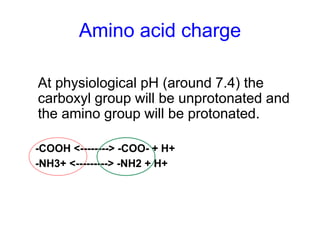
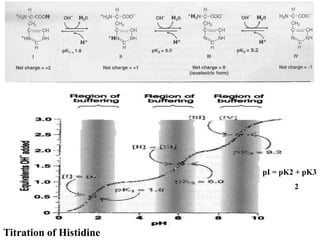
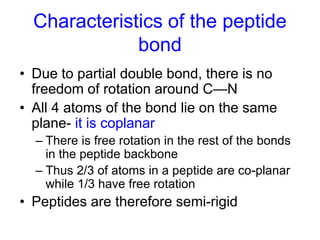
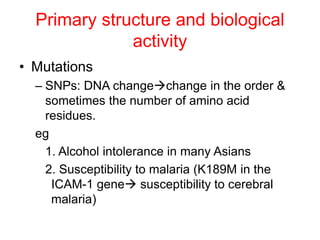
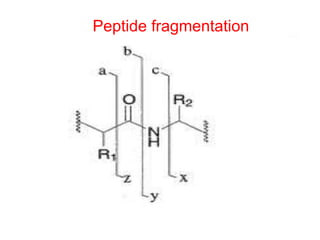
The document discusses amino acids and their properties. It introduces the 20 standard amino acids that make up mammalian proteins, as well as 2 additional amino acids coded by DNA in a non-standard manner. Amino acids have different structures depending on their variable R groups, and can be classified based on properties like polarity, acidity, and charge. Peptides are formed from amino acids joined by peptide bonds. The peptide bond gives peptides some rigidity and planar structure. Important peptides include glutathione, bradykinin, and peptide hormones. The primary structure of a protein refers to the specific sequence of amino acid residues that make it up.