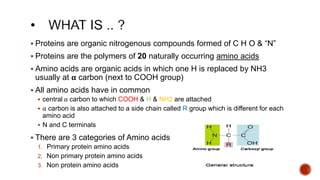
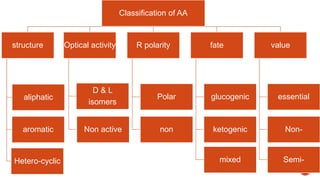
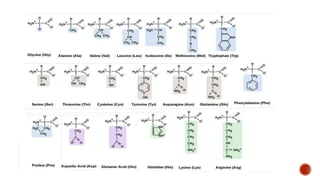
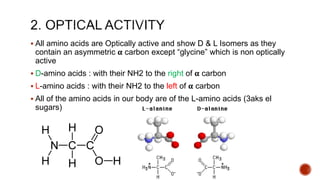
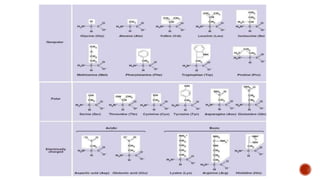
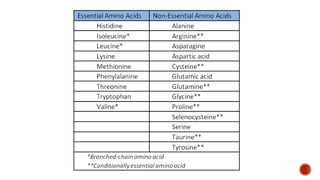
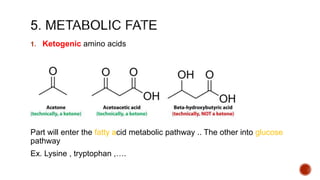
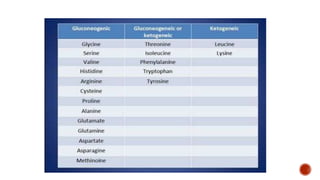
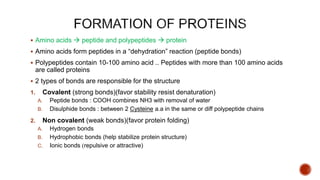
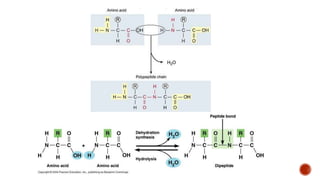
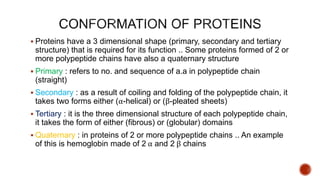
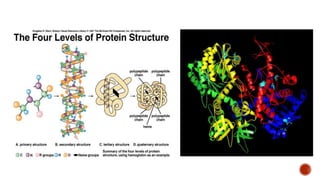
Proteins are composed of amino acids that link together via peptide bonds. There are 20 naturally occurring amino acids that vary in properties like polarity, charge, and ability to form secondary structures. The sequence and interactions of amino acids give proteins their unique 3D structures and functions. Denaturation disrupts non-covalent bonds within proteins, altering their shapes and eliminating biological activity.