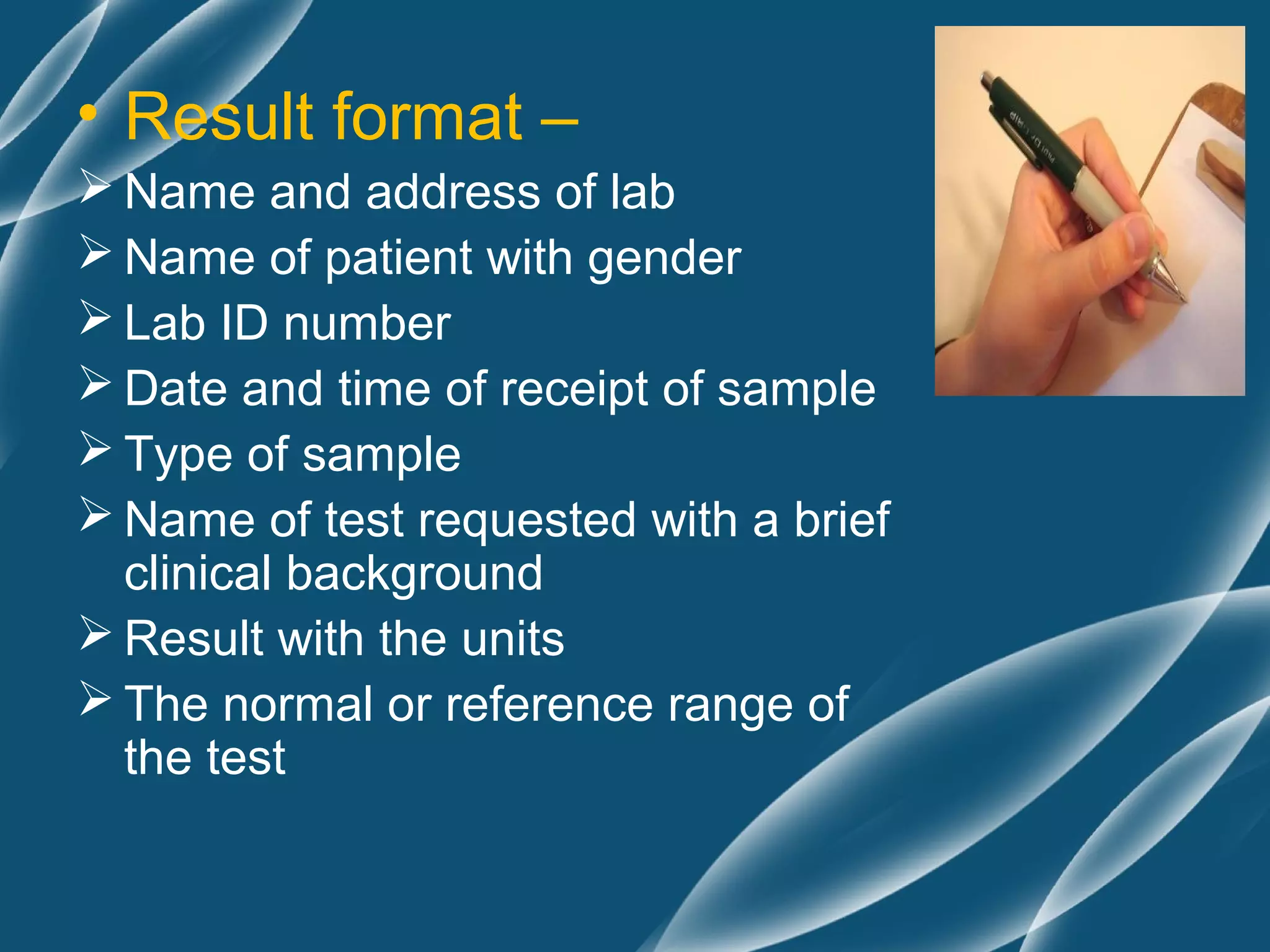
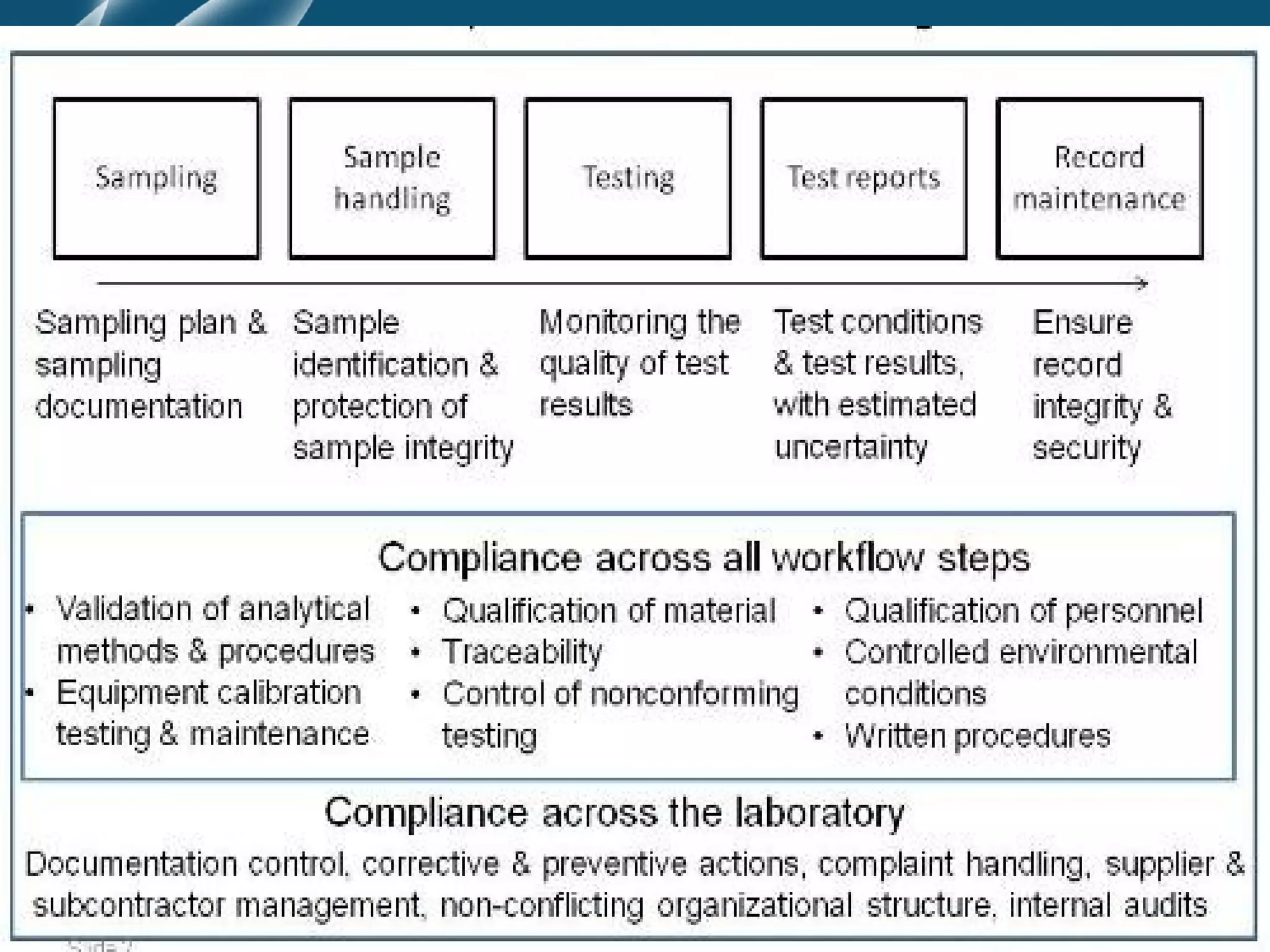
The document discusses principles of pre-analytic, analytic, and post-analytic test management. It covers test selection and evaluation, requisition and test menu formats, and report formatting. The three phases of quality assurance - pre-analytic, analytic, and post-analytic - are described in detail, including factors influencing each phase like specimen handling, equipment calibration, and report review. Quality control procedures are also outlined to ensure test accuracy and reproducibility.





![FACTORS TO BE CONSIDERED BEFORE
PERFORMING TESTS
(i) Direct access testing [DAT]- patients are
health conscious
• No markups or kick backs
• Ethical or social issues attached to reports
• Usually waived
• Doctor X patient relationship ](https://image.slidesharecdn.com/preanalyticandpostanalytictestmanagement-121122142838-phpapp01/75/Pre-analytic-and-postanalytic-test-management-10-2048.jpg)