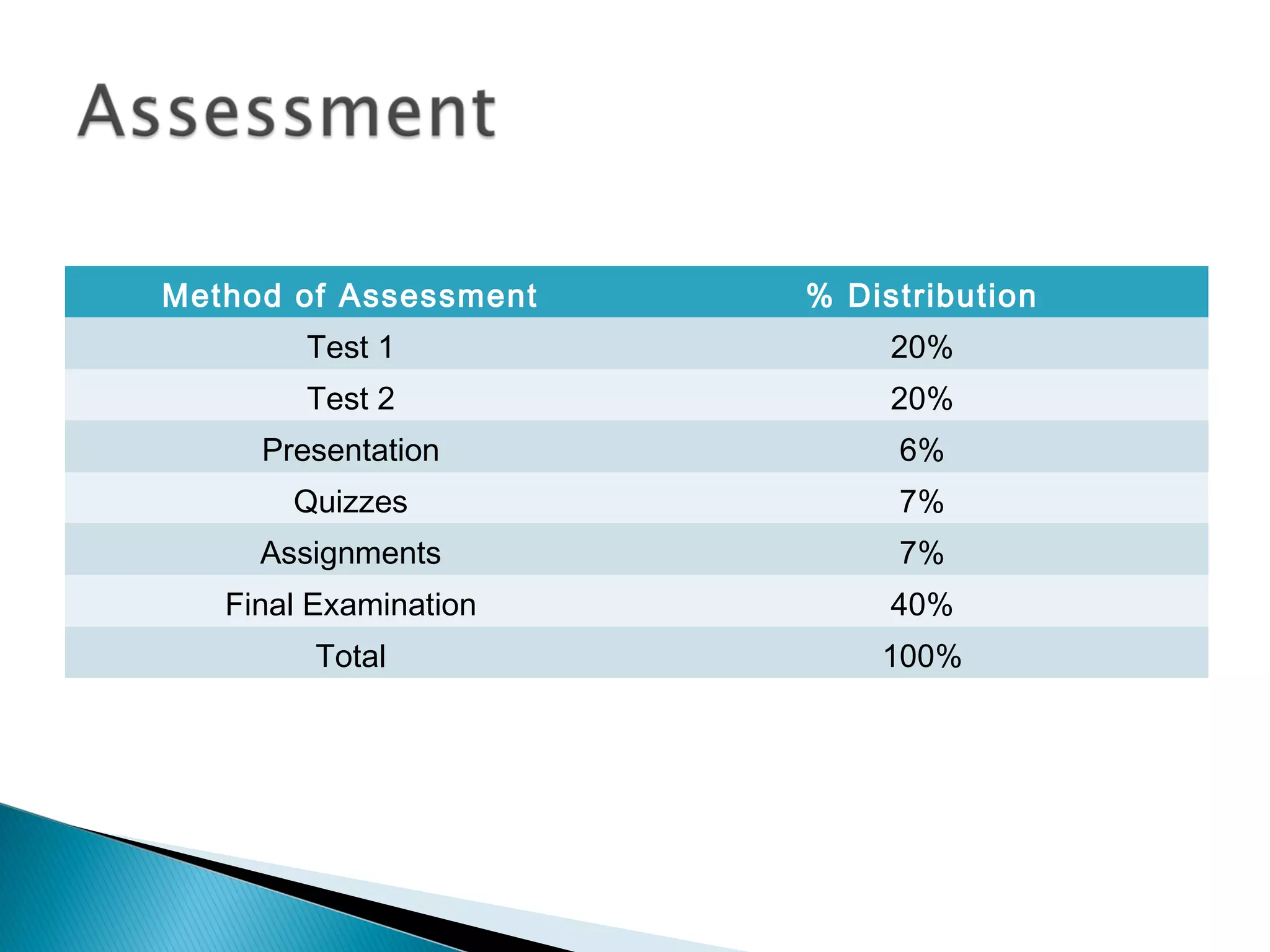
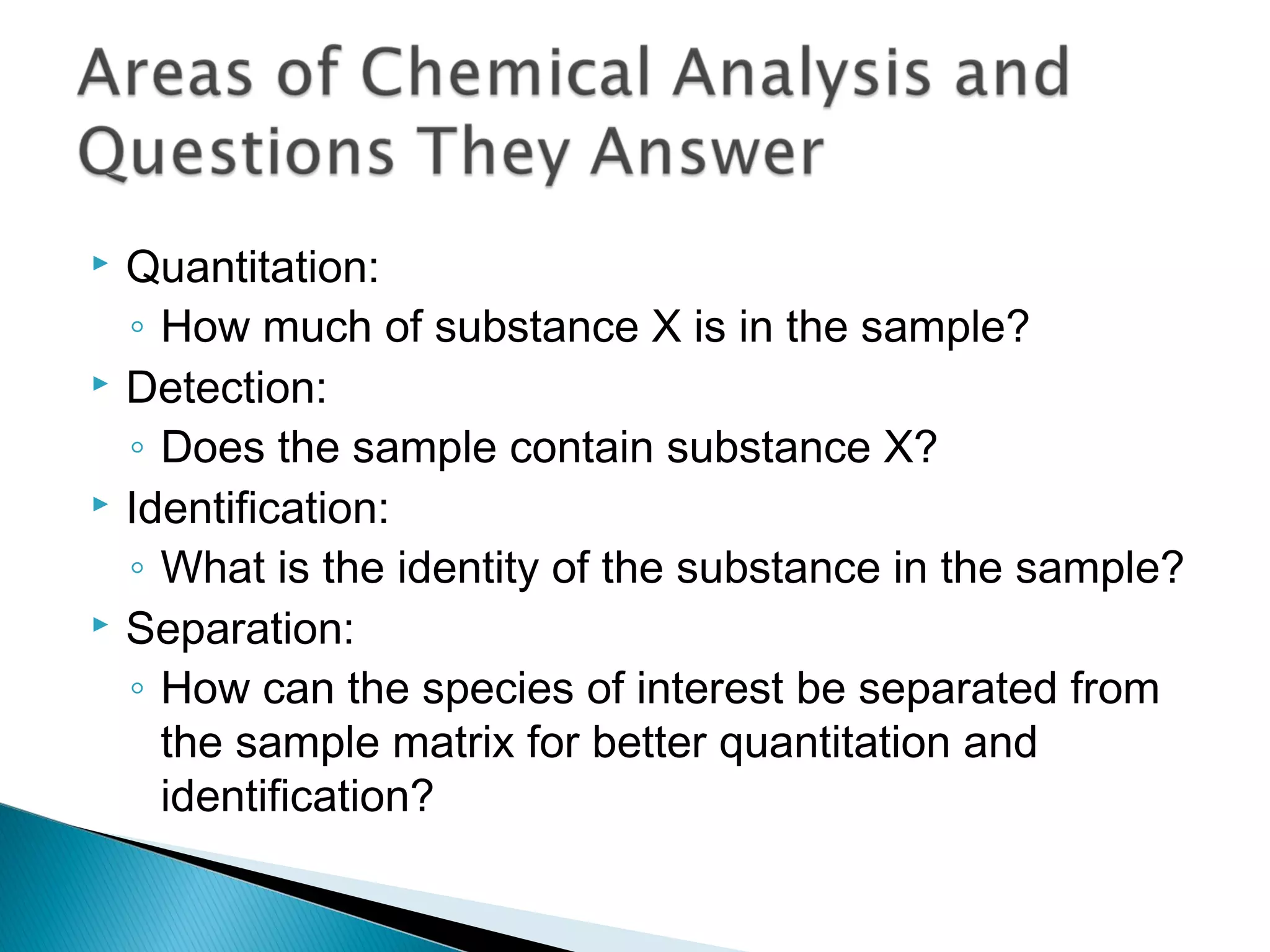
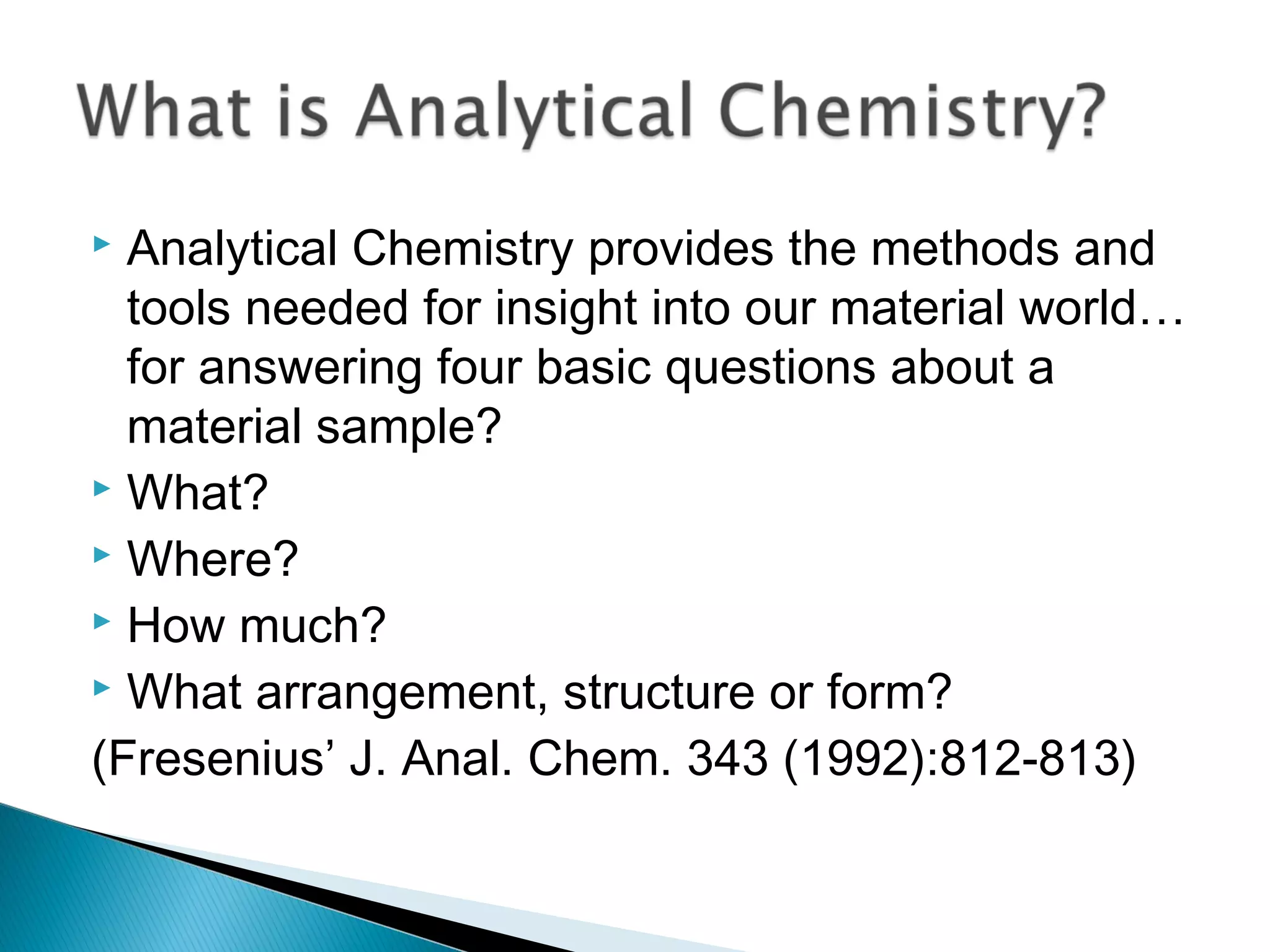
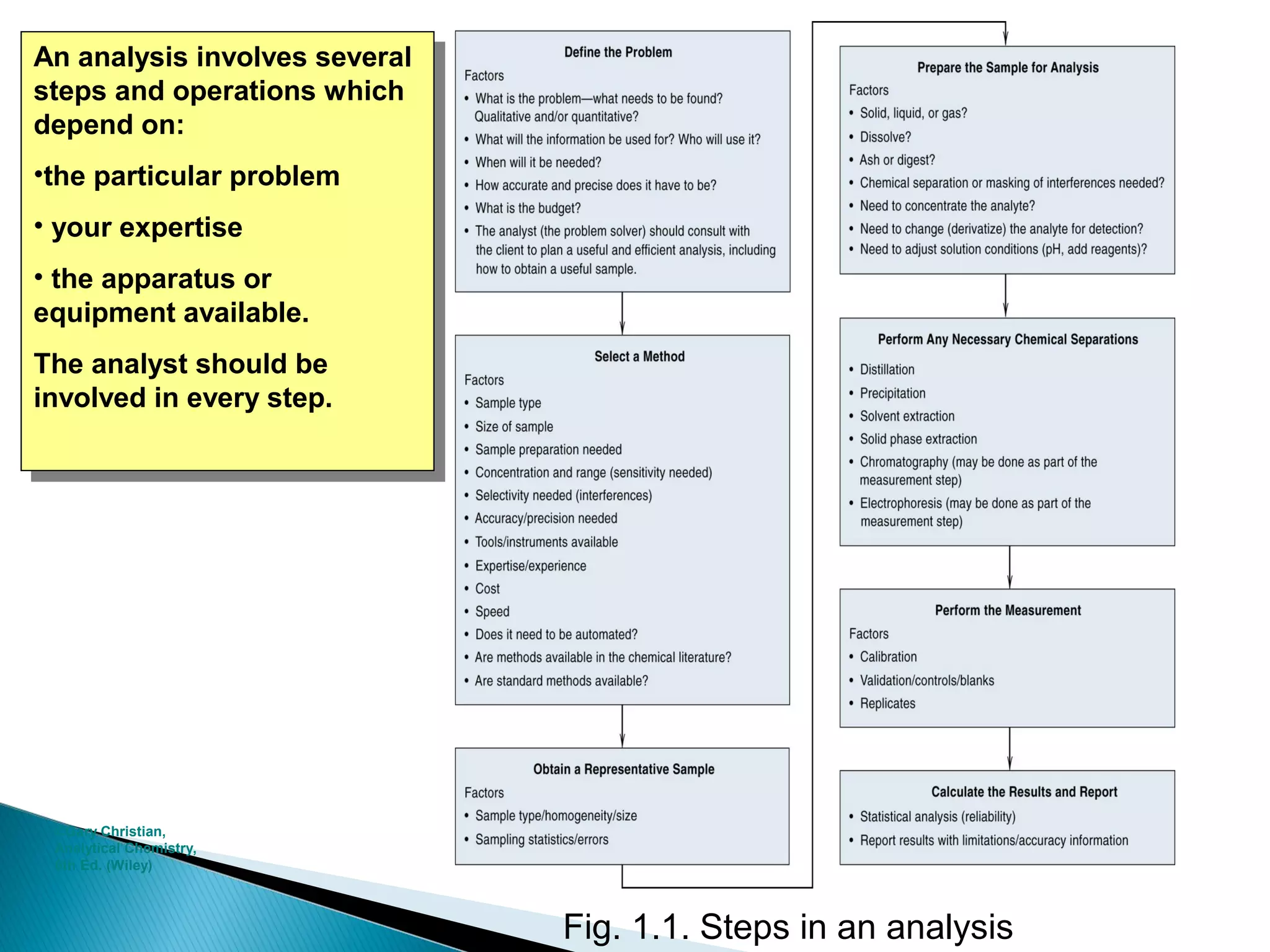
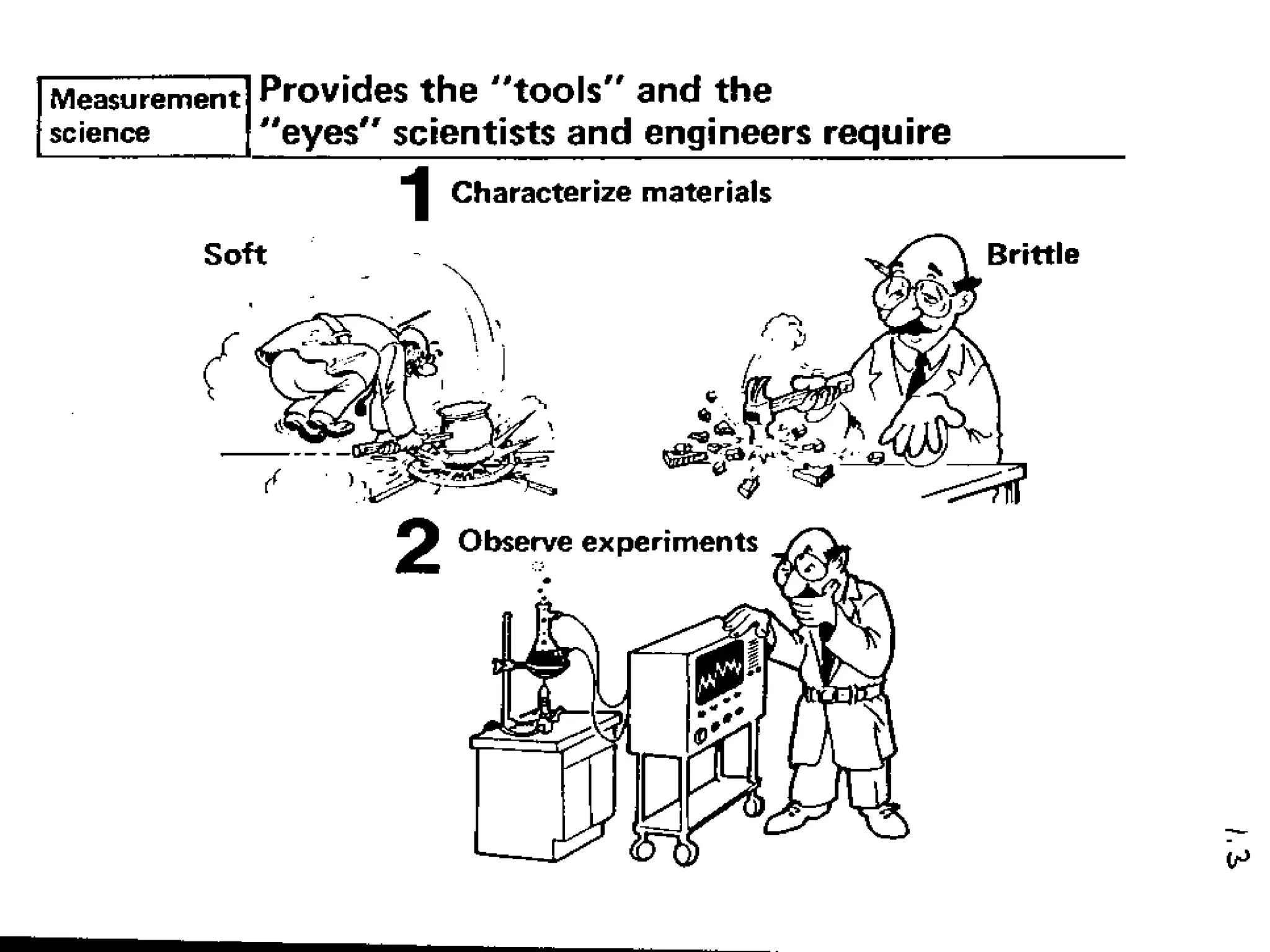
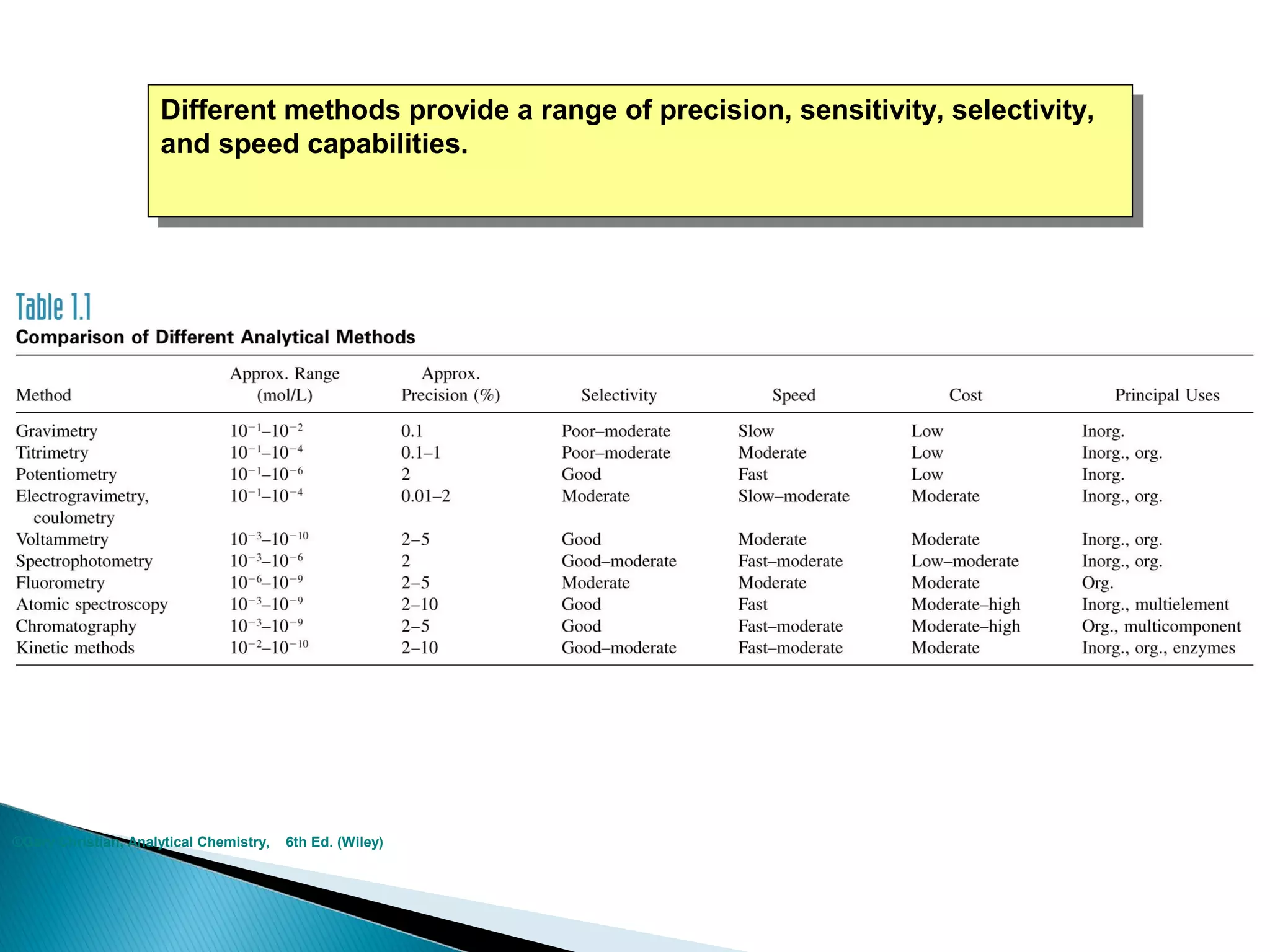
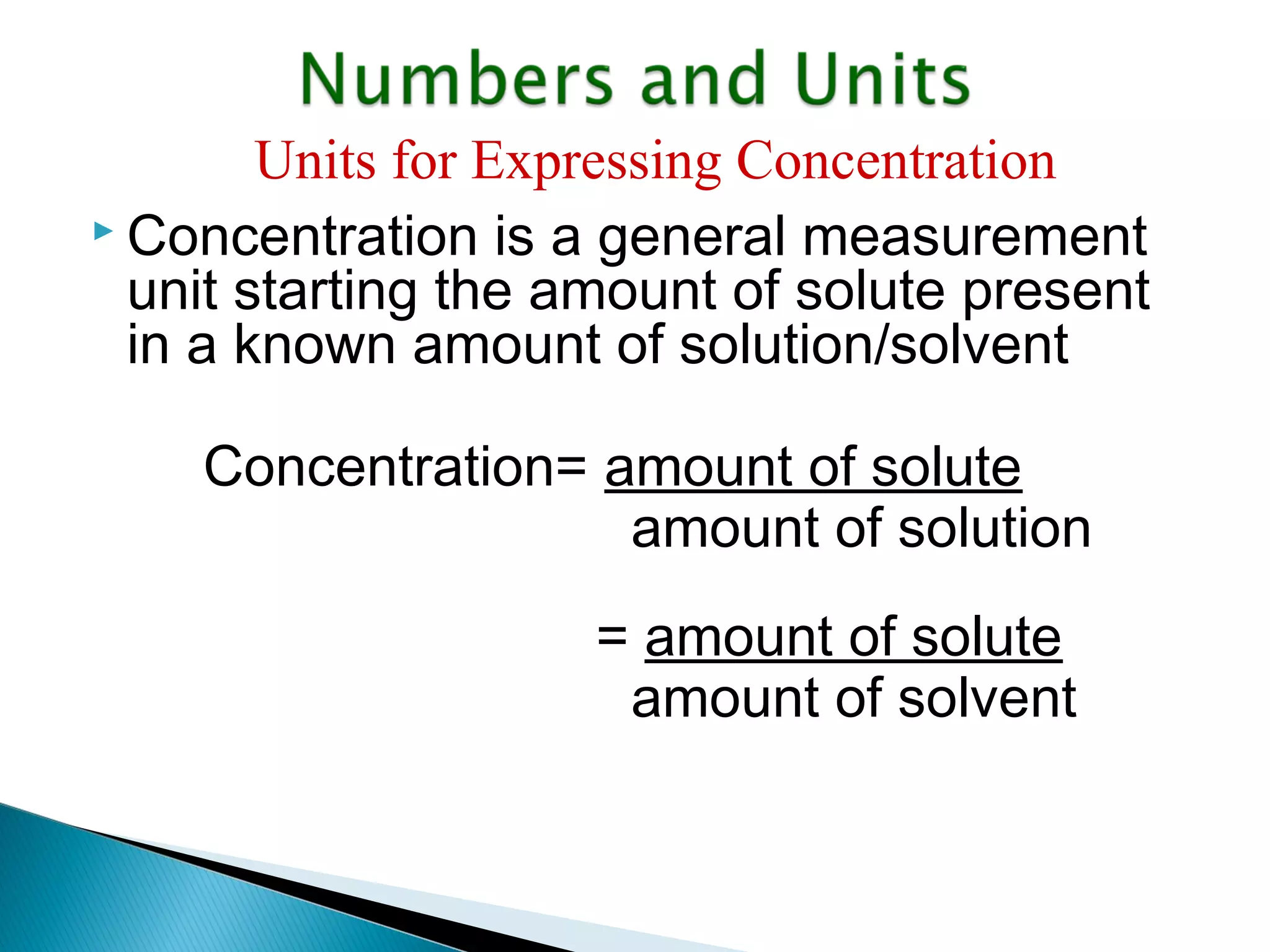
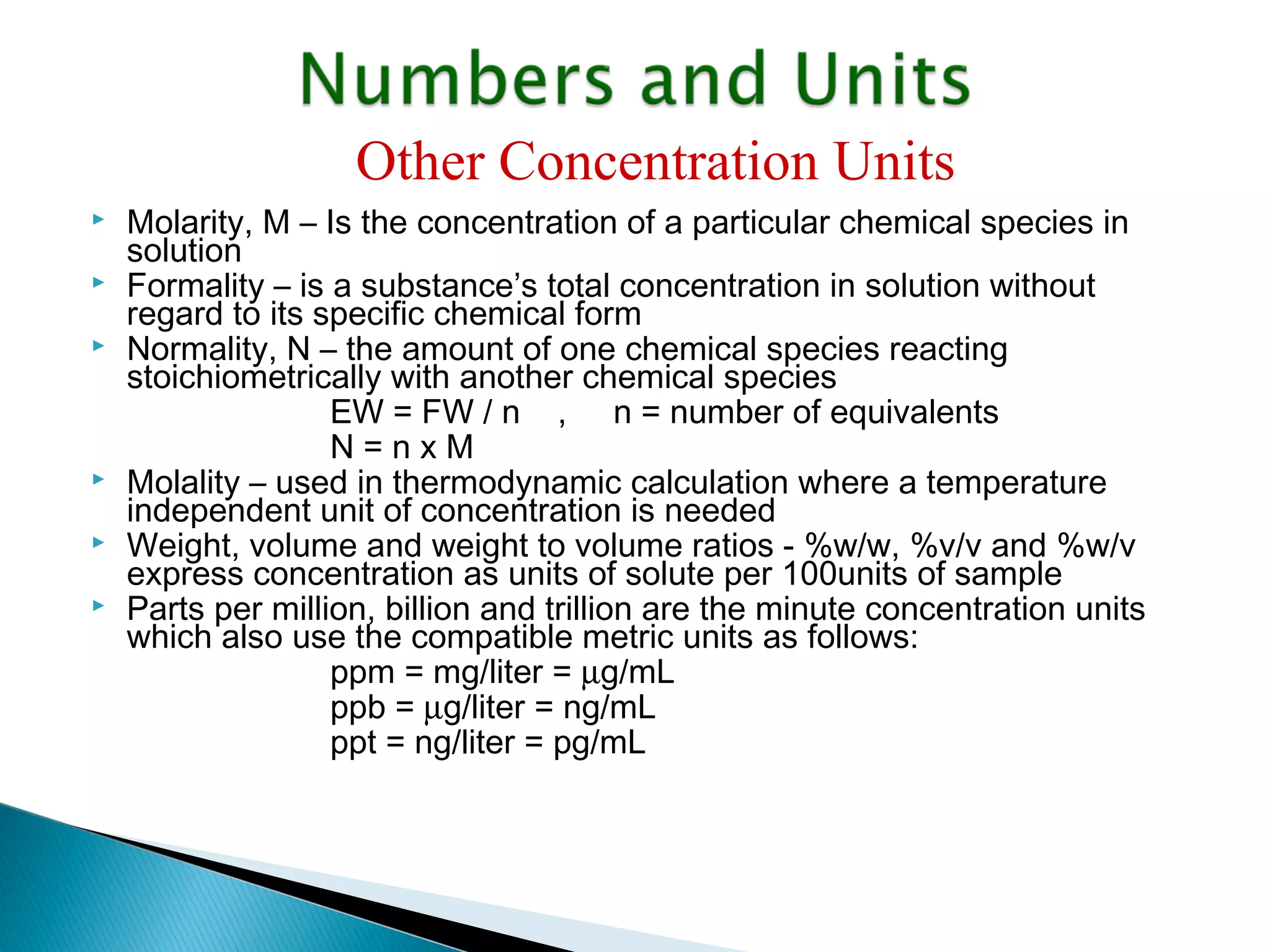
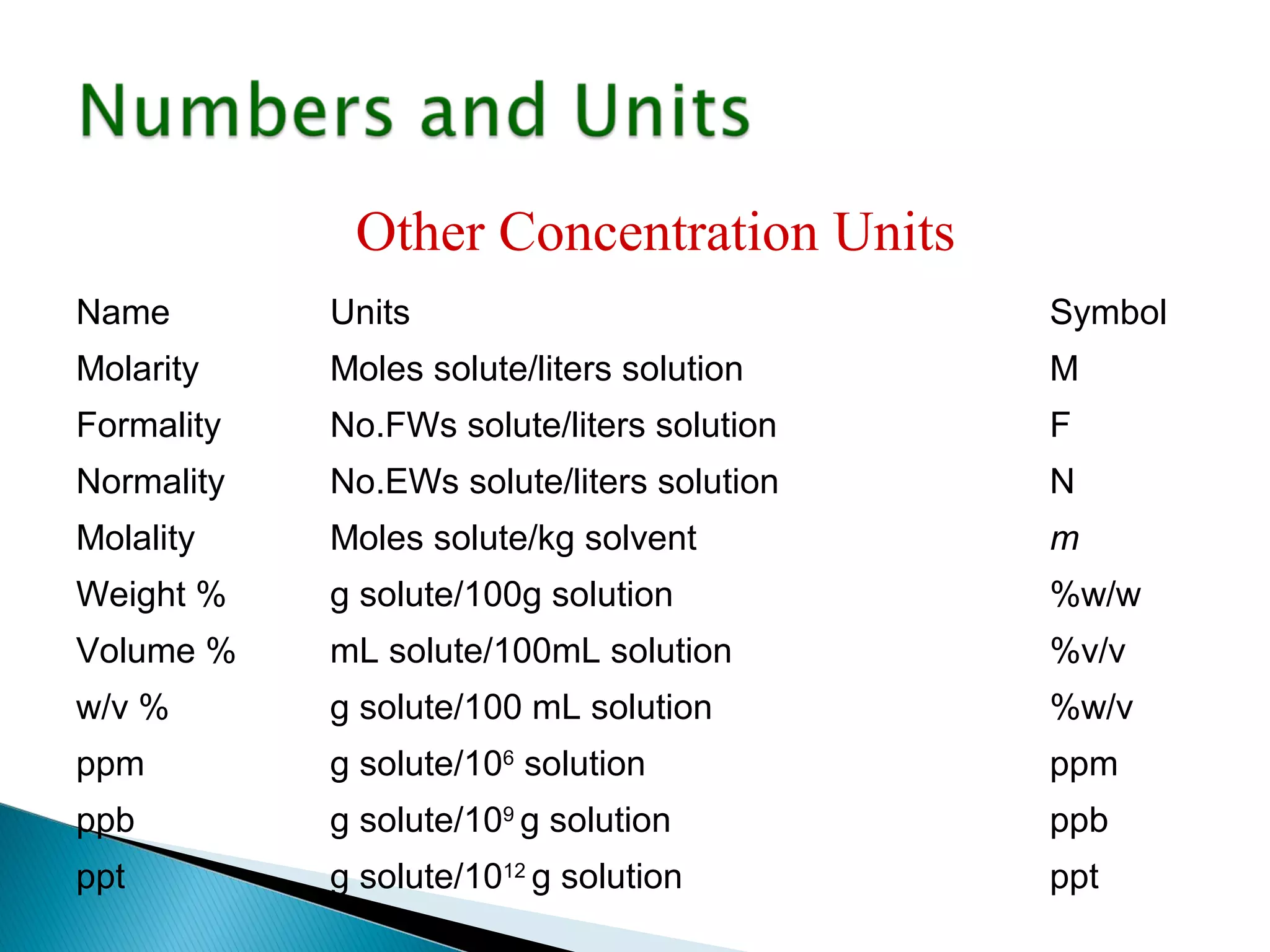
The document provides information about analytical chemistry lectures including the lecturer's contact details, lecture times, assessment breakdown, recommended textbooks, class policies, and an overview of topics to be covered in student presentations which include various analytical techniques such as gravimetric analysis, titrimetry, spectroscopy, chromatography, and mass spectrometry.