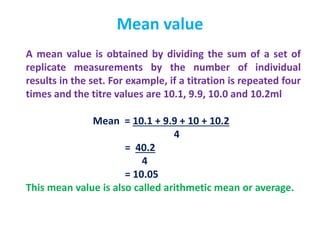
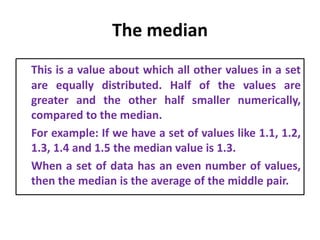
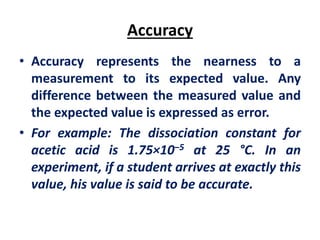
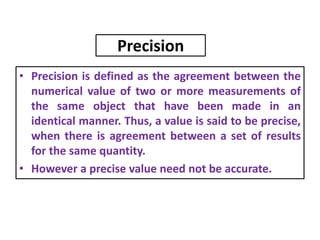
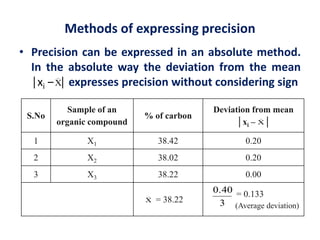
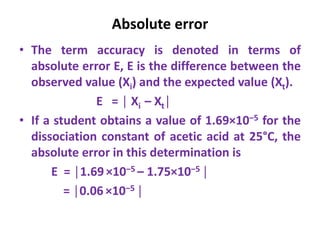
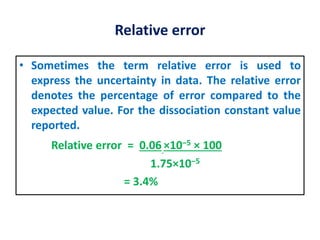
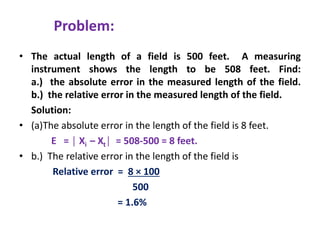
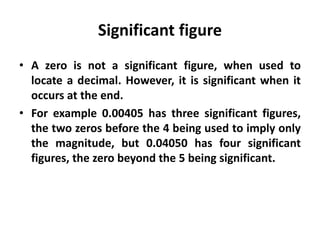
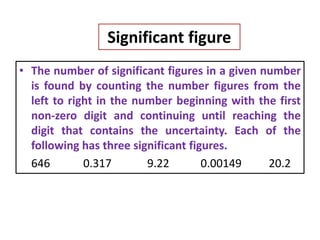
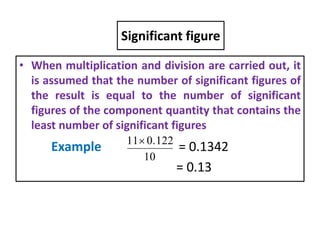
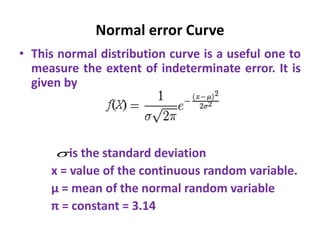
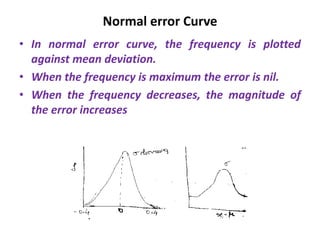
The document provides a comprehensive overview of concepts in analytical chemistry, including mean, median, accuracy, precision, and types of errors in chemical analysis. It distinguishes between determinate (instrumental, operative, methodic) and indeterminate errors while explaining how to calculate absolute and relative errors. Additionally, it addresses the importance of significant figures and the normal error curve in assessing measurement reliability.