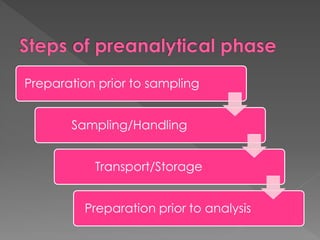
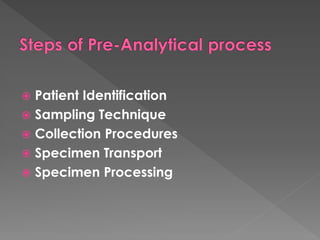
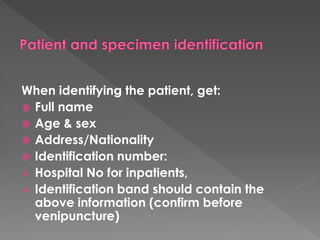
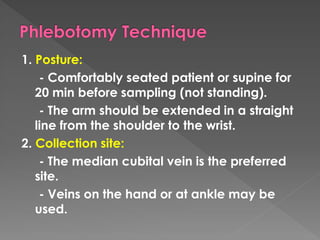
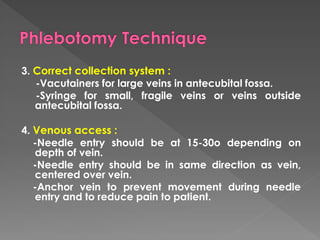
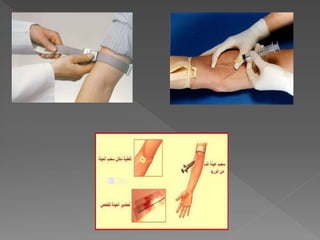
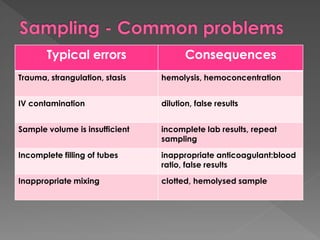
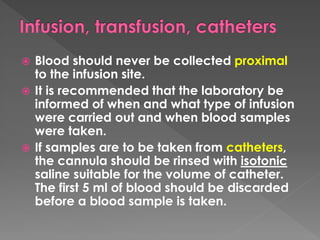
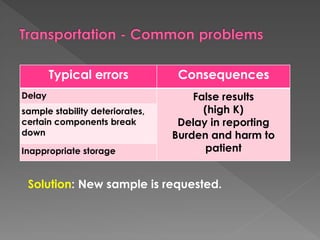
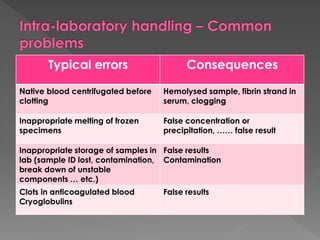
The document discusses the three phases of laboratory testing - pre-analytical, analytical, and post-analytical. It emphasizes that pre-analytical and post-analytical errors account for over 90% of total laboratory errors. Close attention to pre-analytical variables like specimen collection, transport, and storage is critical to ensure accurate test results. Proper procedures and quality control throughout the testing process can prevent many potential errors.